Abstract
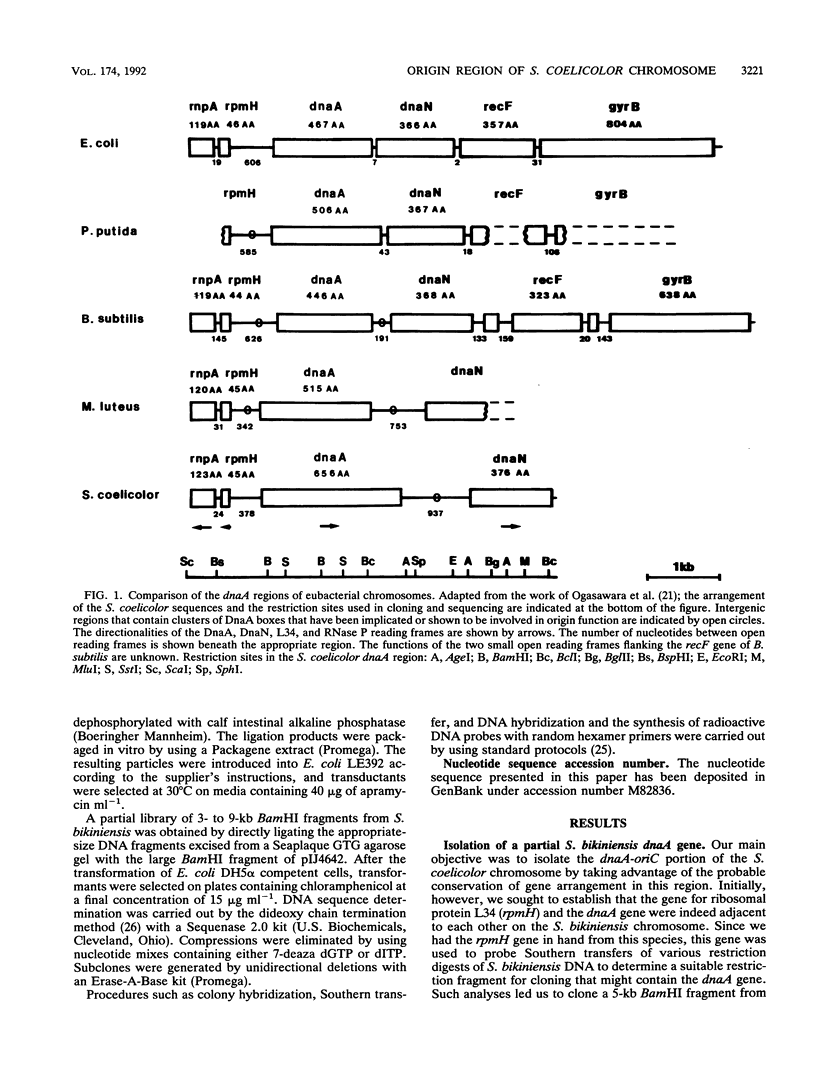
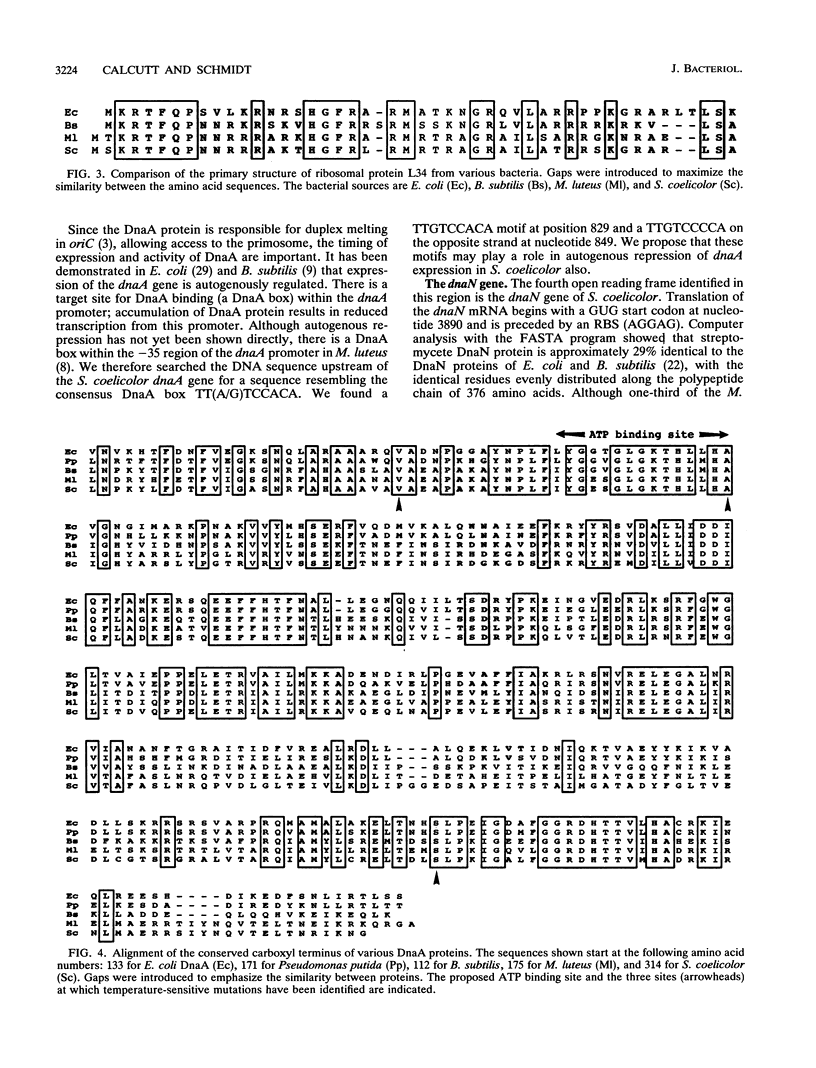
A 23-kb fragment of the Streptomyces coelicolor chromosome spanning the dnaA region has been isolated as a cosmid clone. Nucleotide sequence analysis of a 5-kb portion shows that the genes for the RNase P protein (rnpA), ribosomal protein L34 (rpmH), the replication initiator protein (dnaA), and the beta subunit of DNA polymerase III (dnaN) are present in the highly conserved gene arrangement found in all eubacterial genomes studied so far. The dnaA-dnaN intergenic region is approximately 1 kb and contains a cluster of at least 12 DnaA boxes with a consensus sequence of TTGTCCACA matching the consensus DnaA box in the phylogenetically related Micrococcus luteus. Two DnaA boxes precede the dnaA sequence. We propose that the chromosomal origin (oriC) of S. coelicolor lies between dnaA and dnaN. In related work, J. Zakrzewska-Czerwinska and H. Schrempf (J. Bacteriol. 174:2688-2693, 1992) have identified the homologous sequence from the closely-related Streptomyces lividans as capable of self-replication.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benigni R., Petrov P. A., Carere A. Estimate of the genome size by renaturation studies in Streptomyces. Appl Microbiol. 1975 Aug;30(2):324–326. doi: 10.1128/am.30.2.324-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Micrococcus luteus: conservation and variations among eubacteria. Gene. 1990 Sep 1;93(1):73–78. doi: 10.1016/0378-1119(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Pseudomonas putida: conservation among three bacteria, Bacillus subtilis, Escherichia coli and P. putida. Mol Gen Genet. 1989 Feb;215(3):381–387. doi: 10.1007/BF00427033. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. Physical mapping and nucleotide sequence of the rnpA gene that encodes the protein component of ribonuclease P in Escherichia coli. Gene. 1985;38(1-3):85–93. doi: 10.1016/0378-1119(85)90206-9. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S., Kornberg A. A novel protein binds a key origin sequence to block replication of an E. coli minichromosome. Cell. 1990 Oct 19;63(2):325–331. doi: 10.1016/0092-8674(90)90165-b. [DOI] [PubMed] [Google Scholar]
- Kieser T., Melton R. E. Plasmid pIJ699, a multi-copy positive-selection vector for Streptomyces. Gene. 1988 May 15;65(1):83–91. doi: 10.1016/0378-1119(88)90419-2. [DOI] [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Motamedi H., Lee K., Nichols L., Schmidt F. J. An RNA species involved in Escherichia coli ribonuclease P activity. Gene cloning and effect on transfer RnA synthesis in vivo. J Mol Biol. 1982 Dec 15;162(3):535–550. doi: 10.1016/0022-2836(82)90387-4. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., von Meyenburg K., Hansen F. G., Yoshikawa H. Conservation of genes and their organization in the chromosomal replication origin region of Bacillus subtilis and Escherichia coli. EMBO J. 1985 Dec 1;4(12):3345–3350. doi: 10.1002/j.1460-2075.1985.tb04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Richardson M. A., Kuhstoss S. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 1987;153:166–198. doi: 10.1016/0076-6879(87)53053-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vioque A., Arnez J., Altman S. Protein-RNA interactions in the RNase P holoenzyme from Escherichia coli. J Mol Biol. 1988 Aug 20;202(4):835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. Transcriptional repression of the dnaA gene of Escherichia coli by dnaA protein. Mol Gen Genet. 1987 Oct;209(3):518–525. doi: 10.1007/BF00331158. [DOI] [PubMed] [Google Scholar]
- Zakrzewska-Czerwińska J., Schrempf H. Characterization of an autonomously replicating region from the Streptomyces lividans chromosome. J Bacteriol. 1992 Apr;174(8):2688–2693. doi: 10.1128/jb.174.8.2688-2693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



