Abstract
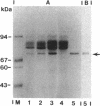
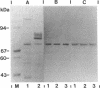
A substantial portion of the second peptidoglycan hydrolase (muramidase-2) activity of Enterococcus hirae ATCC 9790 (formerly Streptococcus faecium) is present in the supernatant culture medium. In contrast, nearly all muramidase-1 activity is associated with cells in the latent, proteinase-activatable form. Muramidase-2 activity is produced and secreted throughout growth, with maximal levels attained at or near the end of exponential growth in a rich organic medium. Muramidase-2 activity in the culture medium remained high even during overnight incubations in the absence of proteinase inhibitors. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of supernatant culture medium concentrated by 60% saturated ammonium sulfate precipitation showed the presence of several Coomassie blue-staining bands. One intensely staining protein band, at about 71 kDa, selectively adsorbed to the insoluble peptidoglycan fraction of cell walls of E. hirae, retained muramidase-2 activity, and reacted in Western immunoblots with monoclonal antibodies to muramidase-2. The mobility of extracellular muramidase-2 in sodium dodecyl sulfate-polyacrylamide gel electrophoresis was indistinguishable from that of muramidase-2 extracted with 6 M guanidine hydrochloride from intact bacteria. Muramidase-2 appears to have only a limited number of binding sites on the peptidoglycan of E. hirae cell walls but binds with high affinity. Although high levels of muramidase-2 activity were present in supernatants of stationary-phase cultures, the bacteria were resistant to autolysis. Thus it appears that the peptidoglycan in walls of intact cells of E. hirae is somehow protected from the hydrolytic action of extracellular muramidase-2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. F., Dolinger D. L., Schramm V. L., Shockman G. D. The mechanism of soluble peptidoglycan hydrolysis by an autolytic muramidase. A processive exodisaccharidase. J Biol Chem. 1984 Oct 10;259(19):11818–11827. [PubMed] [Google Scholar]
- Barrett J. F., Shockman G. D. Isolation and characterization of soluble peptidoglycan from several strains of Streptococcus faecium. J Bacteriol. 1984 Aug;159(2):511–519. doi: 10.1128/jb.159.2.511-519.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. P., Kariyama R., Daneo-Moore L., Shockman G. D. Cloning and sequence analysis of the muramidase-2 gene from Enterococcus hirae. J Bacteriol. 1992 Mar;174(5):1619–1625. doi: 10.1128/jb.174.5.1619-1625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Salton M. R. Solubility characteristics of Micrococcus lysodeikticus membrane components in detergents and chaotropic salts analyzed by immunoelectrophoresis. Biochim Biophys Acta. 1979 May 3;553(1):40–53. doi: 10.1016/0005-2736(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Cornett J. B., Johnson C. A., Shockman G. D. Release of autolytic enzyme from Streptococcus, faecium cell walls by treatment with dilute alkali. J Bacteriol. 1979 Jun;138(3):699–704. doi: 10.1128/jb.138.3.699-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Daneo-Moore L., Shockman G. D. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J Bacteriol. 1989 Aug;171(8):4355–4361. doi: 10.1128/jb.171.8.4355-4361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Schramm V. L., Shockman G. D. Covalent modification of the beta-1,4-N-acetylmuramoylhydrolase of Streptococcus faecium with 5-mercaptouridine monophosphate. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6667–6671. doi: 10.1073/pnas.85.18.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Horowitz P. M., Simon D. The enzyme rhodanese can be reactivated after denaturation in guanidinium chloride. J Biol Chem. 1986 Oct 25;261(30):13887–13891. [PubMed] [Google Scholar]
- Jackson D. E., Wong W., Largen M. T., Shockman G. D. Monoclonal antibodies to immunodeterminants of lipoteichoic acids. Infect Immun. 1984 Mar;43(3):800–803. doi: 10.1128/iai.43.3.800-803.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Autolytic formation of protoplasts (autoplasts) of Streptococcus faecalis; location of active and latent autolysin. J Bacteriol. 1976 Sep;127(3):1482–1493. doi: 10.1128/jb.127.3.1482-1493.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyama R., Massidda O., Daneo-Moore L., Shockman G. D. Properties of cell wall-associated DD-carboxypeptidase of Enterococcus hirae (Streptococcus faecium) ATCC 9790 extracted with alkali. J Bacteriol. 1990 Jul;172(7):3718–3724. doi: 10.1128/jb.172.7.3718-3724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCoy L. F., Jr, Wong K. P. Renaturation of bovine erythrocyte carbonic anhydrase B denatured by acid, heat, and detergent. Biochemistry. 1981 May 26;20(11):3062–3067. doi: 10.1021/bi00514a012. [DOI] [PubMed] [Google Scholar]
- Mendoza J. A., Rogers E., Lorimer G. H., Horowitz P. M. Unassisted refolding of urea unfolded rhodanese. J Biol Chem. 1991 Jul 25;266(21):13587–13591. [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell H., Facklam R. R. Guanidine extraction of streptococcal M protein. Infect Immun. 1975 Sep;12(3):679–686. doi: 10.1128/iai.12.3.679-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein C. H. Solubility as a function of protein structure and solvent components. Biotechnology (N Y) 1990 Apr;8(4):308–317. doi: 10.1038/nbt0490-308. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderich B., Höltje J. V. Subcellular distribution of the soluble lytic transglycosylase in Escherichia coli. J Bacteriol. 1991 Sep;173(18):5668–5676. doi: 10.1128/jb.173.18.5668-5676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]