Abstract
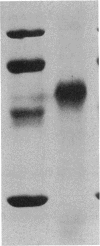
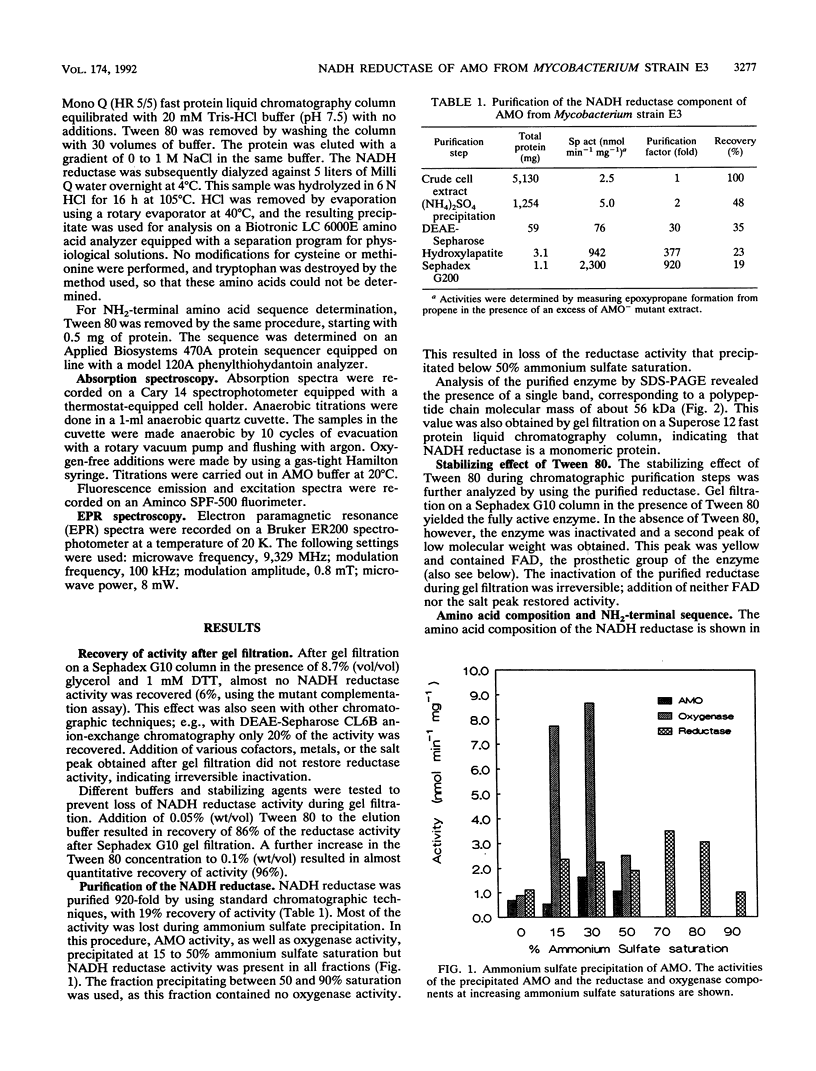
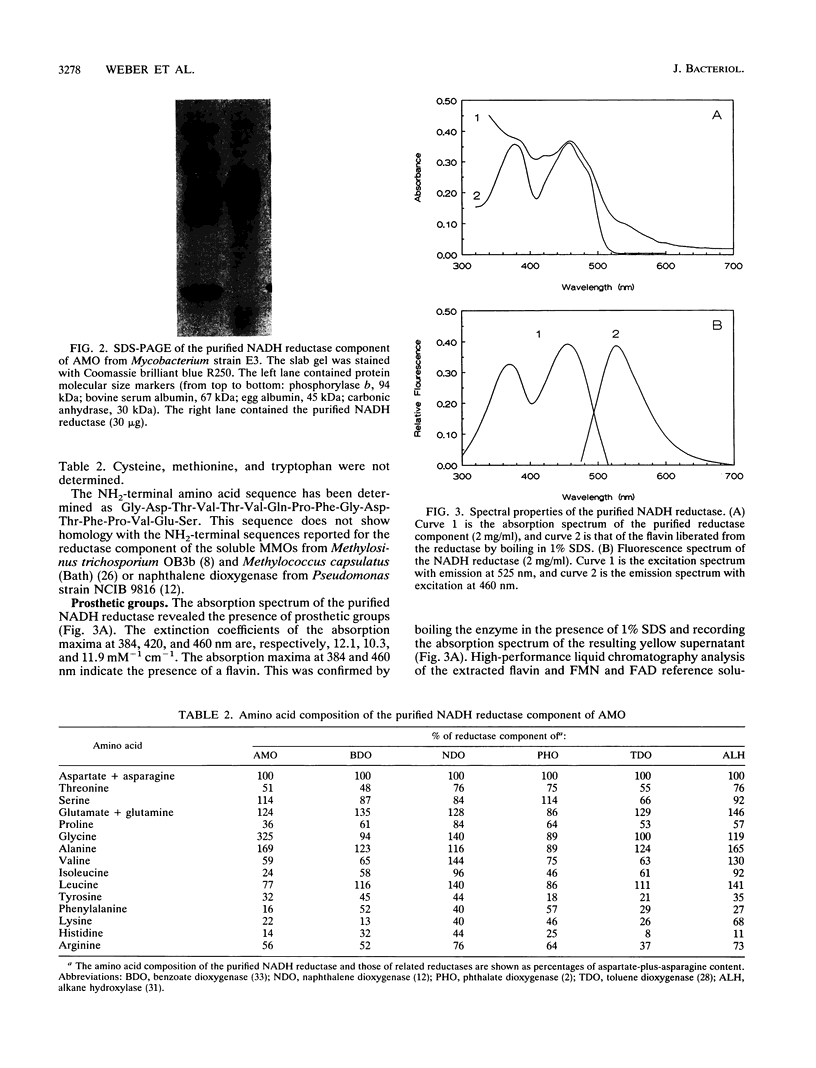
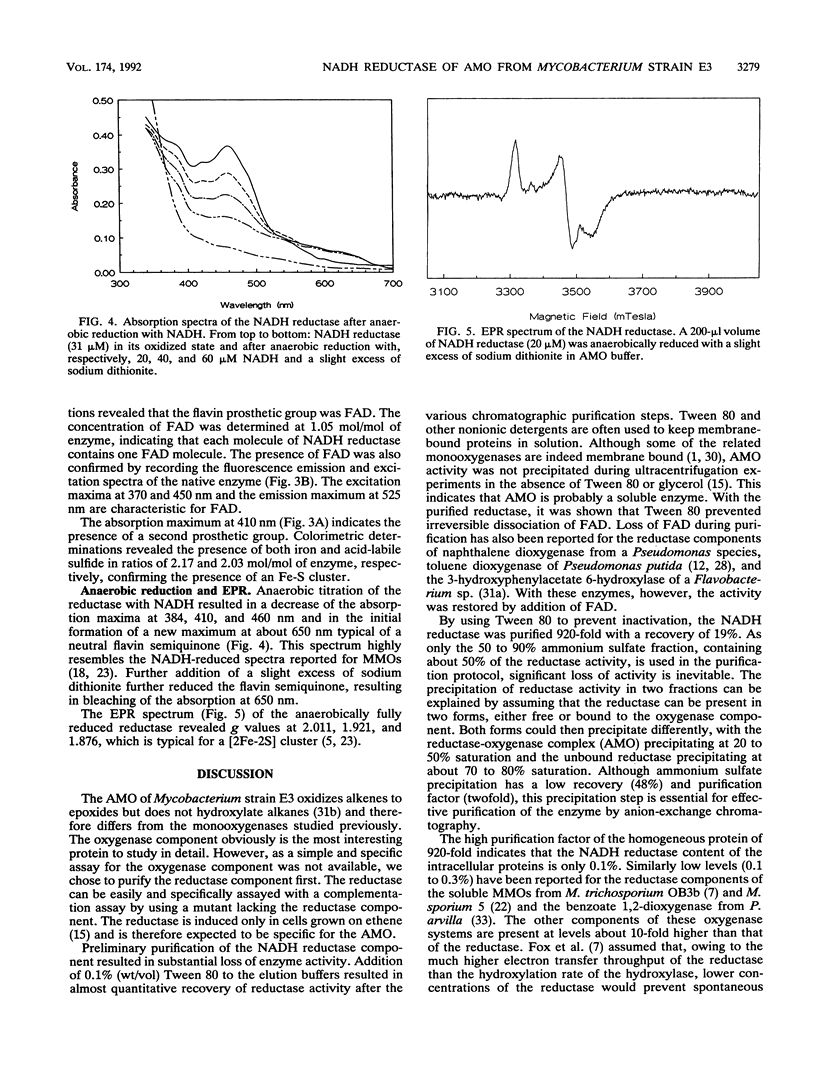
Alkene monooxygenase, a multicomponent enzyme system which catalyzes the epoxidation of short-chain alkenes, is induced in Mycobacterium strain E3 when it is grown on ethene. We purified the NADH reductase component of this enzyme system to homogeneity. Recovery of the enzyme was 19%, with a purification factor of 920-fold. The enzyme is a monomer with a molecular mass of 56 kDa as determined by gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It is yellow-red with absorption maxima at 384, 410, and 460 nm. Flavin adenine dinucleotide (FAD) was identified as a prosthetic group at a FAD-protein ratio of 1:1. Tween 80 prevented irreversible dissociation of FAD from the enzyme during chromatographic purification steps. Colorimetric analysis revealed 2 mol each of iron and acid-labile sulfide, indicating the presence of a [2Fe-2S] cluster. The presence of this cluster was confirmed by electron paramagnetic resonance spectroscopy (g values at 2.011, 1.921, and 1.876). Anaerobic reduction of the reductase by NADH resulted in formation of a flavin semiquinone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUMBY P. E., MILLER R. W., MASSEY V. THE CONTENT AND POSSIBLE CATALYTIC SIGNIFICANCE OF LABILE SULFIDE IN SOME METALLOFLAVOPROTEINS. J Biol Chem. 1965 May;240:2222–2228. [PubMed] [Google Scholar]
- Batie C. J., LaHaie E., Ballou D. P. Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia. J Biol Chem. 1987 Feb 5;262(4):1510–1518. [PubMed] [Google Scholar]
- Bernhardt F. H., Pachowsky H., Staudinger H. A 4-methoxybenzoate O-demethylase from Pseudomonas putida. A new type of monooxygenase system. Eur J Biochem. 1975 Sep 1;57(1):241–256. doi: 10.1111/j.1432-1033.1975.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzymatic -oxidation. VI. Isolation of homogeneous reduced diphosphopyridine nucleotide-rubredoxin reductase. J Biol Chem. 1972 Apr 10;247(7):2109–2116. [PubMed] [Google Scholar]
- Fox B. G., Froland W. A., Dege J. E., Lipscomb J. D. Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph. J Biol Chem. 1989 Jun 15;264(17):10023–10033. [PubMed] [Google Scholar]
- Fox B. G., Liu Y., Dege J. E., Lipscomb J. D. Complex formation between the protein components of methane monooxygenase from Methylosinus trichosporium OB3b. Identification of sites of component interaction. J Biol Chem. 1991 Jan 5;266(1):540–550. [PubMed] [Google Scholar]
- Geary P. J., Saboowalla F., Patil D., Cammack R. An investigation of the iron-sulphur proteins of benzene dioxygenase from Pseudomonas putida by electron-spin-resonance spectroscopy. Biochem J. 1984 Feb 1;217(3):667–673. doi: 10.1042/bj2170667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmans S., Weber F. J., Somhorst D. P., de Bont J. A. Alkene monooxygenase from Mycobacterium: a multicomponent enzyme. J Gen Microbiol. 1991 Nov;137(11):2555–2560. doi: 10.1099/00221287-137-11-2555. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund J., Woodland M. P., Dalton H. Electron transfer reactions in the soluble methane monooxygenase of Methylococcus capsulatus (Bath). Eur J Biochem. 1985 Mar 1;147(2):297–305. doi: 10.1111/j.1432-1033.1985.tb08750.x. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Reductive titrations of iron-sulfur proteins containing two to four iron atoms. J Biol Chem. 1969 Nov 25;244(22):6143–6148. [PubMed] [Google Scholar]
- Patel R. N. Methane monooxygenase: purification and properties of flavoprotein component. Arch Biochem Biophys. 1987 Jan;252(1):229–236. doi: 10.1016/0003-9861(87)90027-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Purification and reconstitution of the electron transport components for 6-deoxyerythronolide B hydroxylase, a cytochrome P-450 enzyme of macrolide antibiotic (erythromycin) biosynthesis. J Bacteriol. 1988 Apr;170(4):1548–1553. doi: 10.1128/jb.170.4.1548-1553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainthorpe A. C., Lees V., Salmond G. P., Dalton H., Murrell J. C. The methane monooxygenase gene cluster of Methylococcus capsulatus (Bath). Gene. 1990 Jul 2;91(1):27–34. doi: 10.1016/0378-1119(90)90158-n. [DOI] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Subramanian V., Liu T. N., Yeh W. K., Narro M., Gibson D. T. Purification and properties of NADH-ferredoxinTOL reductase. A component of toluene dioxygenase from Pseudomonas putida. J Biol Chem. 1981 Mar 25;256(6):2723–2730. [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M., Wada K., Kobayashi H. Estimation of labile sulfide in iron-sulfur proteins. Anal Biochem. 1975 Oct;68(2):632–636. doi: 10.1016/0003-2697(75)90659-4. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel W. J., Van Den Tweel W. J. Purification and characterisation of 3-hydroxyphenylacetate 6-hydroxylase: a novel FAD-dependent monooxygenase from a Flavobacterium species. Eur J Biochem. 1991 Nov 1;201(3):585–592. doi: 10.1111/j.1432-1033.1991.tb16318.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Fujisawa H. Characterization of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla c-1. J Biol Chem. 1978 Dec 25;253(24):8848–8853. [PubMed] [Google Scholar]