Abstract
It is controversial whether the endothelial cell release of nitric oxide (NO) or a different factor(s) accounts for endothelium-dependent hyperpolarization, because in many arteries endothelium-dependent relaxation and hyperpolarization resists inhibitors of NO synthase. The contribution of NO to acetylcholine-induced endothelium-dependent hyperpolarization and relaxation of the rabbit carotid artery was determined by measuring NO with electrochemical and chemiluminescence techniques. In the presence of phenylephrine to depolarize and contract the smooth muscle cells, acetylcholine caused concentration-dependent hyperpolarization and relaxation which were closely correlated to the release of NO. Nω-nitro-l-arginine methyl ester (30 μM) partially reduced the release of NO and caused a similar reduction in smooth muscle cell relaxation and hyperpolarization. To determine if the residual responses were mediated by another endothelium-derived mediator or NO released despite treatment with Nω-nitro-l-arginine methyl ester, Nω-nitro-l-arginine (300 μM) was added. The combined inhibitors further reduced, but did not eliminate, NO release, smooth muscle relaxation, and hyperpolarization. Hyperpolarization and relaxation to acetylcholine remained closely correlated with the release of NO in the presence of the inhibitors. In addition, the NO donor, SIN-1, caused hyperpolarization and relaxation which correlated with the concentrations of NO that it released. These studies indicate that (i) the release of NO by acetylcholine is only partially inhibited by these inhibitors of NO synthase when used even at high concentrations, and (ii) NO rather than another factor accounts fully for endothelium-dependent responses of the rabbit carotid artery.
Keywords: endothelium-derived hyperpolarizing factor, acetylcholine, SIN-1, Nω-nitro-l-arginine, Nω-nitro-l-arginine methyl ester
Nitric oxide (NO) is now accepted to be the major mediator of endothelium-dependent, arterial smooth muscle relaxation (1). However, whether or not NO accounts for endothelium-dependent hyperpolarization is controversial (2, 3). Authentic NO and NO donors hyperpolarize vascular smooth muscle cells (4); however, in some studies, concentrations of NO that were too high to be considered physiological were required, or the hyperpolarization observed was smaller than that caused by an endothelial cell agonist (2, 3). In addition, in many in vitro and in vivo studies of human and animal arteries, endothelium-dependent relaxation, vasodilatation, and hyperpolarization persist in the presence of l-arginine analogues that are inhibitors of NO synthase (NOS) (5–20). This has led to the supposition that factors other than NO are important mediators of endothelium-dependent responses including hyperpolarization (2, 3). Cytochrome P450 metabolites of arachidonic acid (21, 22), and carbon monoxide (23) have been proposed as alternative mediators. Neither the release from the endothelium of sufficient quantities of these mediators to account for the responses has been measured, nor has NO release been measured directly to exclude its contribution. This is despite the fact that the release of NO from endothelial cells may not be completely blocked by the NOS inhibitors (24, 25) in the concentrations that have been used to exclude its role. In the present study of the rabbit carotid artery, NO was directly measured by two independent techniques to examine its role in the endothelium-dependent relaxation and hyperpolarization to acetylcholine (ACh). In this blood vessel, high concentrations of the NOS inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME), do not fully block endothelium-dependent relaxation and hyperpolarization (7, 26).
METHODS
The carotid arteries were removed from New Zealand White rabbits (2–3 kg), cleaned of adhering fat and connective tissue, and cut into cylindrical segments.
Solutions and Drugs.
Experiments were carried out in physiological buffer as described (27, 28). Indomethacin (1 μM) was present in all solutions to exclude prostaglandins as a potential mediator. Drugs used were purchased from Sigma, except 3-morpholinosydnonimine hydrochloride (SIN-1) which was obtained from Alexis (San Diego).
Microsensor Measurements of NO Released by ACh and SIN-1.
NO was measured electrochemically with a porphyrinic microsensor (29, 30). The NO microsensor was produced by threading a single carbon fiber (Amoco Performance Products, Greenville, NC) through a pulled end of an L-shaped glass capillary, with a 6.0-mm length of the fiber left protruding. A copper wire was inserted into the opposite end of the glass capillary which was sealed with conductive silver epoxy (AI Technology, Lawrenceville, NJ). Then the tip of the glass capillary was sealed with beeswax. The active carbon fiber tip of the porphyrinic sensor was made more sensitive to NO and less sensitive to potential interfering substances by the cyclic voltammetric deposition (−0.20 to 1.00 V at 100 mV/s for 10 cycles) of highly conductive polymeric porphyrin from a solution of 0.25 mM nickel (II) tetrakis (3-methoxy-4-hydroxyphenyl)porphyrin (NiTMHPP) in 0.1 M NaOH. After drying, the electrode tip was coated by dipping (three times for 4 s each) in 1% Nafion in alcohol (Aldrich), which produced a thin anionic film that repelled or retarded charged species while allowing small neutral and hydrophobic NO access to the underlying catalytic surface. Linear calibration curves were constructed for each sensor from 2 × 10−9 to 2 × 10−5 M NO, before and after in vitro measurements, using aliquots of saturated NO prepared as described (31). The microsensor had a response time of 0.1 ms at micromolar NO concentrations and 10 ms at the detection limit of 1 nM.
For measurements of NO released from endothelium by ACh, carotid arteries were cut open into 3-mm segments and pinned with the endothelium face up in a small Petri dish coated with Sylgard containing 0.5 ml physiological buffer, incubated with or without NO inhibitors. Measurements were done 60 min after the animal was killed. The buffer in the dish was gassed with 95% O2/5% CO2 at 37°C for 30 min; bubbling was stopped 30–60 s before measurement. The Petri dish was placed under a microscope, and a single fiber porphyrinic sensor (diameter 5 ± 1 μm) was positioned with the help of a computer-controlled micromanipulator (X, Y, Z resolution, 0.2 μm) and CD camera. The tip of the electrode was slowly lowered to the endothelial cell surface until a current was detected due to the piezoelectric effect associated with cell contact (5–10 pA, 4–7 ms duration). From that position the sensor was then retracted 2 microns from the cell surface with the micromanipulator. Amperometry or differential pulse voltammetry (DPV) were performed using a Princeton Applied Research model 273 voltammetric analyzer interfaced with IBM 8086 computer with data acquisition and control software. DPV was used to measure the changes of NO concentration with time (response time 0.1 ms). In both the amperometric and the DPV method a three electrode system was used with a porphyrinic sensor (working electrode), saturated calomel electrode (reference electrode), and platinum electrode (auxiliary electrode).
NO concentration (nM) was measured after adding single concentrations of ACh (10−8 to 3 × 10−6 M) to individual arterial rings. Because the porphyrinic sensor only detects NO in the immediate vicinity of the electrode surface, the concentration of NO measured was a local rather than a bulk concentration. The concentration of NO released by ACh decreased exponentially with distance from the cell surface. At a distance of 40–50 μm from the cell membrane the NO concentration was usually undetectable by the sensor. Peak concentration of NO occurring in the first few seconds after stimulation is reported because the gradient of concentration and therefore the accuracy of the measurement is greatest at this time. After this, concentration falls exponentially so that after 3 min, values reach ≈30% of those initially measured.
The bulk concentration of NO released in solution by SIN-1 was measured directly in the buffer with a multifiber porphyrinic sensor (four fibers, 10-μm diameter). The concentration of NO increased with time and reached a stable plateau after 4 min. This plateau concentration of NO measured for each concentration of SIN-1 is reported here.
Measurements of Nitrite Release Caused by ACh.
Nitrite release was measured by adapting previous methods (25, 32) and using a Siever’s Instrument (Boulder, CO) chemiluminescence NO analyzer (model 270B) to estimate the cumulative release of NO from the arteries released during a concentration response to ACh (25, 32). Carotid arteries were cut into 1-cm segments and four to six segments were placed in a test tube containing 5 ml buffer gassed with 95% O2/5% CO2 gas mixture at 37°C. The buffer was changed every 30 min for 90 min. The rings then were placed in 1 ml of buffer for 30 min so that basal accumulation of nitrite formed from released NO could be measured. The buffer was then replaced, and the rings were challenged over a 30-min period with half-log increments in the concentration of ACh (10−8 to 3 × 10−6 M). Increasing concentrations of ACh were administered every 5 min, timed to mimic the administration to single arterial rings in which tension and membrane potential were measured. NOS inhibitors were added 30 min before addition of ACh, and maintained subsequently. Rings were challenged only once with ACh. At the end of each 30-min period, 0.25 ml of the buffer was injected into the purge vessel of the chemiluminescence analyzer that held glacial acetic acid containing 1% potassium iodide at room temperature as reducing agent. A stream of nitrogen gas passed the NO formed from nitrite in the purge vessel into a reaction chamber where it reacted with ozone to produce light, generating a signal from a photomultiplier that was integrated by a line crossing recorder. Measurements were calibrated with authentic sodium nitrite standards. The detection limit of the instrument for nitrite was 2 pmol; the basal release of nitrite from the arteries was ≈3-fold higher than this detection limit. The arterial rings were cut open, the endothelial cell surface area was measured, and the results expressed as pmol NO per cm2 intimal surface area.
Under these conditions, ACh caused a measurable increase in the release of nitrite into the buffer. The 90-min preincubation period was found to be important to reduce the basal overflow of nitrogen oxides to levels at which the ACh-stimulated levels became significant. In addition, background nitrite levels were reduced as much as possible by freshly preparing physiological salt solution and rinsing glassware with dionized water (Milli-Q; Millipore). Preliminary studies showed that the release of nitrite stimulated by ACh did not occur if the endothelium was removed by rubbing and could be blocked by atropine. Thirty-minute basal accumulation of nitrite in control rings averaged 30 ± 6.1 pmol/cm2, which increased to 190 ± 33 pmol/cm2 after ACh stimulation (n = 11). Levels did not change from basal in the latter 30-min collection if ACh was excluded, and they were unaffected by endothelial cell removal or the addition of NOS inhibitors. Thus, basal accumulation of nitrite presumably represents overflow from the tissue of preformed nitrite. Results are presented as the level of nitrite after the 30-min concentration response to ACh minus the preceding 30-min basal value. Nitrite, rather than total nitrogen oxides, was measured in these studies for two reasons. First, stronger reducing agents used to measure both nitrate and nitrite also reduced the nitro-arginine groups of the arginine analogue inhibitors of NOS forming large amounts of NO and preventing detection of NO from the endothelium. These particular inhibitors were employed because they have been used most extensively in previous physiological studies. Second, although NO released from tissues is partially oxidized to nitrates, nitrite represents a significant component of the nitrogen oxides recovered after release from the endothelium stimulated by ACh. Preliminary measurements of both nitrite (measured using acetic acid/potassium iodide at room temperature as reducing agent) and total nitrogen oxides (measured using hydrochloric acid and vanadium chloride at 90°C) showed that nitrogen oxides released under basal conditions from carotid arteries consisted of only 10% nitrite (32 ± 7 vs. 257 ± 96 pmol/cm2, nitrite vs. nitrate, n = 11). During ACh stimulation, the additional nitrogen oxides released from the arteries above the basal values were 33% nitrite (172 ± 39 vs. 345 ± 145 pmol/cm2, n = 11). Although it is not known whether the proportion of nitrite and nitrate is altered in the presence of the NOS inhibitors used in this study, the decreased accumulation of nitrite in the medium during ACh stimulation in the presence of NOS inhibitors correlated closely with the decrease of NO release measured with the porphyrinic sensor, suggesting that the proportion of nitrite in total nitrogen oxides remains similar in the presence of the inhibitors.
Measurement of Tension and Membrane Potential.
For simultaneous measurement of isometric force and smooth muscle membrane potential, arteries were mounted in a two-channel myograph (model 400A; J. P. Trading, Aarhus, Denmark) as described (28, 33). The arteries were maintained at 37°C and superfused at 7–8 ml/min with physiological buffer that had been bubbled with 95% O2/5% CO2 gas. Measurement of smooth muscle membrane potential was made with a glass microelectrode advanced through the adventitial surface of the artery as described (28, 34).
Drugs were added to the perfusate before it entered the tissue chamber. The l-arginine analogs were added 20 min before contraction of the artery to a stable level with phenylephrine to ≈50% of maximal tension, and 40 min before adding half-logarithmic concentrations of ACh (10−8–10−5 M) to the chamber. The concentration of phenylephrine reported in the text used to contract the arteries was decreased in arteries treated with l-NAME and Nω-nitro-l-arginine (l-NNA), and was chosen according to previous experiments so that similar tone was achieved under all experimental conditions (7).
Analysis of Data.
Relaxations are expressed as the percentage decrease in tone induced by the presence of phenylephrine. Changes in membrane potential are expressed in mV. The hyperpolarization caused by ACh or SIN-1 is reported as a percentage of the initial depolarization induced by phenylephrine. The values of the initial depolarization are reported in the text. Data are expressed as mean ± SEM for n observations, where n equals the number of individual rabbits from which arteries were taken. The pD2 was calculated as the negative logarithm of the concentration causing 50% smooth muscle relaxation or hyperpolarization; concentrations are reported in the text as the negative logarithm. The significance of differences between mean values was calculated by Student’s t test, with rejection of the null hypothesis at the 5% level. Statistics for concentration-response curves are the result of t tests performed on the responses to the highest concentration of ACh.
RESULTS
Release of NO and Nitrite Caused by ACh.
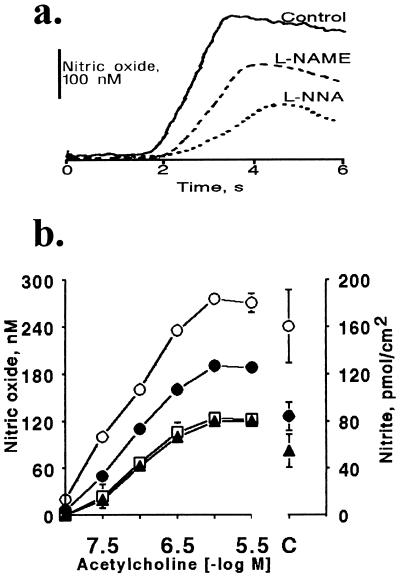
Fig. 1a shows tracings of typical amperometric recordings (current calibrated as NO concentration vs. time) measured with a porphyrinic sensor placed in close proximity to the surface of the endothelium. A short time (2 ± 0.5 s) after injection of ACh (3 × 10−6 M) a rapid and significant increase of NO concentration was observed from its basal level of 8 ± 4 nM (measured with differential pulse voltammetry). The average rate of rise in NO concentration increased significantly to 340 ± 20 nM/s (n = 12). The concentration of NO reached a peak of 270 ± 12 nM after 1.2 s, and then fell exponentially over the next 3 min to a value ≈30% of the peak value. After treatment with l-NAME (3 × 10−5 M) or l-NNA (3 × 10−4 M) the magnitude and kinetics of NO release from endothelial cells changed significantly. The rate of rise of NO concentration induced by ACh (3 × 10−6 M) decreased to 160 ± 20 and 76 ± 8 nM/s (P < 0.005, n = 12, respectively) after treatment with l-NAME or l-NNA. Also the peak concentration of NO released by ACh (3 × 10−6 M) decreased significantly to 188 ± 10 and 122 ± 8 nM after l-NAME or l-NNA treatment (P < 0.005, n = 12, respectively).
Figure 1.
Effect of l-NAME and l-NNA on ACh-induced release of NO and nitrite from rabbit carotid artery. (a) Tracings of typical amperograms obtained with porphyrinic microsensors showing release of NO (nM) caused by ACh (3 × 10−6 M) added at time zero under control conditions, or after treatment with l-NAME (3 × 10−5 M) or l-NNA (3 × 10−4 M, respectively). (b) Plot of peak concentration of NO released in response to each individual ACh concentration (−log M) shown on the left ordinate (n = 12), or cumulative amount of nitrite (pmol/cm2) following the entire concentration response to ACh (10−8–3 × 10−6 M) shown on the right ordinate (C, n = 11). NO release was determined in response to ACh under control conditions (○), or after treatment with l-NAME (3 × 10−5 M; •), l-NNA (3 × 10−4 M; □), or the combination of l-NAME and l-NNA (3 × 10−5 M and 3 × 10−4 M, respectively; ▴). Data are means ± SEM; error bars for NO release in most cases are not visible on the graph due to the size of the symbols.
Fig. 1b shows the peak concentration of NO released by a range of ACh concentrations (10−8–3 × 10−6 M) each administered to individual rings. The NO concentration (nM) rose with increasing concentrations of ACh and reached a maximum at an ACh concentration of 3 × 10−5 M. l-NAME or l-NNA significantly decreased release of NO throughout the concentration response to ACh. No significant synergistic effect was observed for the mixture of l-NAME (3 × 10−5 M) and l-NNA (3 × 10−4 M; Fig. 1b).
After subtracting the basal overflow of nitrite released from carotid artery rings under control conditions (30 ± 6.1 pmol/cm2) from that released during administration of ACh (10−8 to 3 × 10−6 M), the ACh-stimulated release of nitrite was 161 ± 31 pmol/cm2 (n = 11, Fig. 1b). l-NAME (3 × 10−5 M) reduced ACh-stimulated NO release to 84 ± 12 pmol/cm2 above basal levels (Fig. 1b). l-NNA (3 × 10−4 M) and l-NAME (3 × 10−5 M) together significantly reduced NO release to 55 ± 14 pmol/cm2 above basal levels (Fig. 1b).
ACh-Induced Changes in Membrane Potential and Tension.
Smooth muscle cells in isolated segments of carotid artery were electrically quiescent and had a mean resting membrane potential of −58 ± 5.3 mV (75 cells from 38 preparations). Phenylephrine (10−6 M) caused contractions (34 ± 5.8 mN) that were associated with marked depolarization (50 ± 5.6 mV, n = 12, Fig. 2). ACh evoked concentration-dependent smooth muscle relaxation and hyperpolarization (Fig. 2a), neither of which occurred in segments from which the endothelium was removed (data not shown). The maximal relaxation evoked by ACh was 99 ± 1.3%, which was accompanied by a maximal hyperpolarization of the membrane potential to −50 ± 6.2 mV (98 ± 2.6% reversal of the induced depolarization; n = 4, Figs. 2a and 3a). The pD2 values for the relaxation and hyperpolarization to ACh were not significantly different (6.71 ± 0.02 and 6.85 ± 0.04, respectively; n = 4) and the threshold concentration required to initiate both events was the same (3 × 10−8 M).
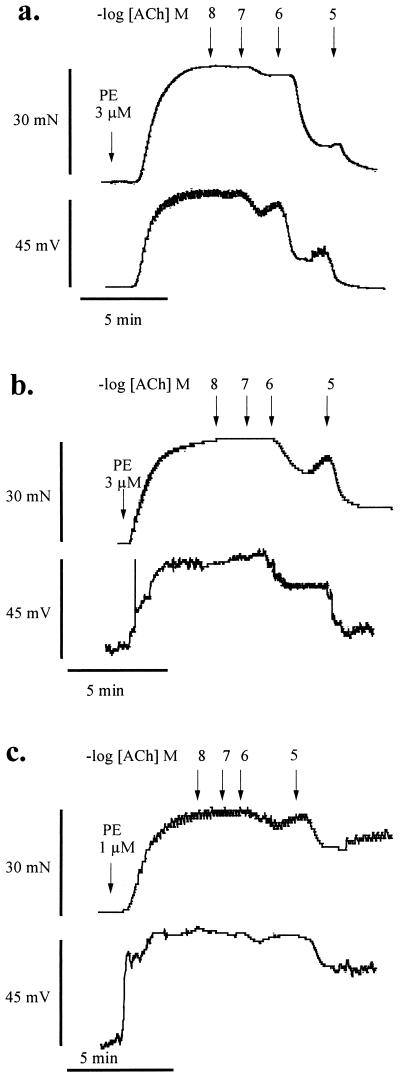
Figure 2.
Effect of l-NAME and l-NNA on ACh-induced relaxation and hyperpolarization. Tracings of recordings of isometric tension (mN) and membrane potential (mV) recorded simultaneously in (a) carotid artery rings under control conditions, (b) treated with l-NAME (3 × 10−5 M), or (c) treated with the combination of l-NAME (3 × 10−5 M) and l-NNA (3 × 10−4 M). The rings were depolarized and contracted with phenylephrine (PE) (1–3 × 10−6 M) and then exposed to cumulative increasing ACh concentrations (−log M) indicated by the arrows.
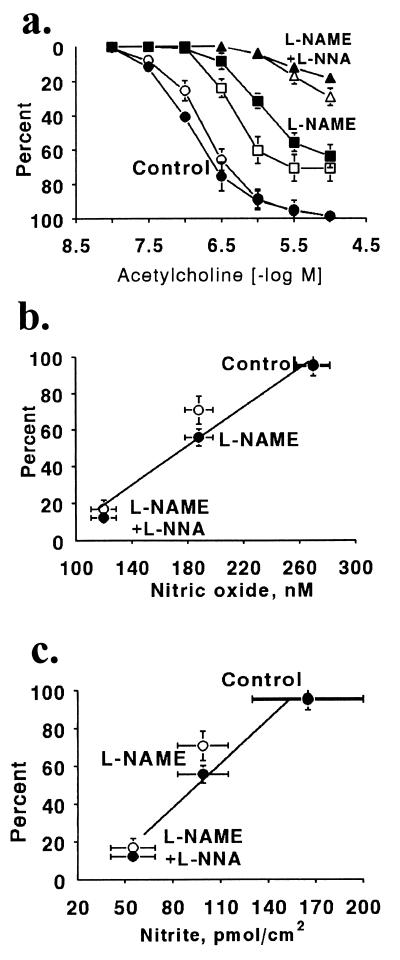
Figure 3.
Effect of l-NAME and l-NNA on ACh-induced hyperpolarization, relaxation, and NO release. ACh-evoked relaxations (open symbols) and hyperpolarizations (filled symbols) recorded from arterial rings contracted and depolarized with phenylephrine under control conditions (circles), after treatment with l-NAME (3 × 10−5 M; squares), or after treatment with l-NAME (3 × 10−5 M) and l-NNA (3 × 10−4 M; triangles) are shown in a. (b) Close correlation between the release of NO measured with the porphyrinic microsensor from rabbit carotid arteries caused by ACh (3 × 10−6 M) under control conditions or after treatment with the same concentrations of l-NAME, or l-NAME combined with l-NNA and the maximal relaxation (filled circles) or hyperpolarization (open circles). (c) Close correlation between the maximal relaxation (filled circles) or hyperpolarization (open circles) to ACh (3 × 10−6 M) and the cumulative release of nitrite caused by the concentration response to ACh (10−8 to 3 × 10−6 M) under control conditions or after treatment with l-NAME alone or with the combination of l-NAME and l-NNA.
Treatment of carotid artery segments with l-NAME (3 × 10−5 M) had no significant effect on basal tone or the resting membrane potential of the smooth muscle cells (Fig. 2b). In addition, the contractions (38 ± 3.1 mN) and depolarization (48 ± 4.3 mV, n = 4) caused by phenylephrine (5 × 10−7 M) were not significantly different from those to phenylephrine (10−6 M) under control conditions. In the presence of l-NAME (3 × 10−5 M), both ACh-evoked relaxation and hyperpolarization of phenylephrine-stimulated arterial segments were significantly inhibited (Figs. 2b and 3a). The maximal relaxation and hyperpolarization were reduced respectively to 71 ± 8.1% and 30 ± 3.6 mV (64 ± 4.7% of the phenylephrine-induced depolarization; n = 4; P < 0.01). Combined treatment with l-NNA (3 × 10−4 M) and l-NAME (3 × 10−5 M) also did not affect basal tension or membrane potential. Phenylephrine (3 × 10−7 M) caused contractions and depolarizations which were similar to those caused by phenylephrine under control conditions (mean contraction and depolarization of 41 ± 1.6 mN and 55 ± 3.3 mV, respectively; n = 4, Fig. 2c). The combined inhibitors further significantly reduced the responses to ACh (Figs. 2c and 3a). In the presence of both inhibitors, the maximal relaxation and hyperpolarization to ACh were reduced respectively to 29 ± 5.0% and 10 ± 2.0 mV (18 ± 2.0% reversal of induced depolarization; n = 4; P < 0.01, Fig. 3a). Simultaneously determined ACh-induced relaxation correlated closely with the ACh-induced hyperpolarization under control conditions as well as after treatment with one or both NOS inhibitors.
The relationship between the simultaneously measured maximal relaxation and hyperpolarization in response to ACh and the release of intact NO or nitrite measured under control conditions, after treatment with l-NAME, or after l-NAME and l-NNA, respectively, is shown in Fig. 3 b and c. Both relaxation and hyperpolarization induced by the highest concentration of ACh correlated closely with either the NO or the accumulated levels of nitrite released into the buffer.
NO Release and Membrane Potential and Tension Changes to SIN-1.
Application of SIN-1 (10−8–10−5 M) to arterial segments contracted with phenylephrine (10−6 M; mean contraction and depolarization of 36 ± 4.8 mN and 50 ± 3.4 mV, respectively; n = 4) evoked concentration-dependent relaxation and hyperpolarization which were highly correlated (Fig. 4a). The maximal relaxation and hyperpolarization evoked by SIN-1 (10−5 M) were 92 ± 4.5% and 48 ± 5.4 mV (97 ± 2.3% reversal of induced depolarization; n = 4), respectively.
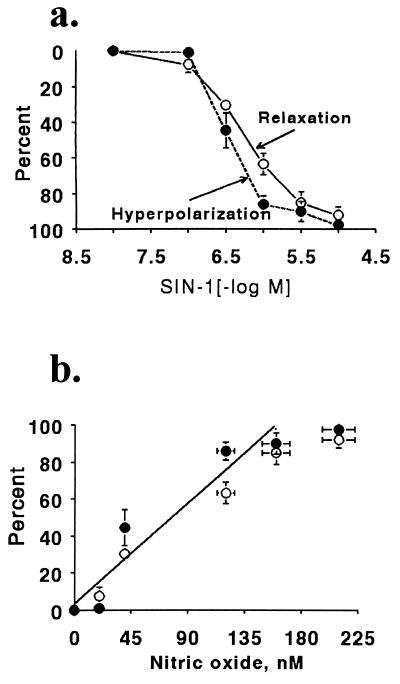
Figure 4.
Relaxation, hyperpolarization, and NO release caused by SIN-1 in rings of carotid artery. (a) Relaxation and hyperpolarization to SIN-1 in arteries contracted to phenylephrine. The values shown are the maximal and stable relaxation attained 4 min after adding each concentration of SIN-1. (b) Correlation between the NO concentration measured with a porphyrinic sensor 4 min after adding SIN-1 to physiological buffer and the relaxation (○) and hyperpolarization (•) caused by each concentration.
The maximal bulk NO concentration generated from SIN-1 (10−5 M) was 210 ± 13 nM. Both the relaxation and hyperpolarization to SIN-1 (10−8–10−5 M) correlated closely with the concentration of NO released into solution by each concentration (Fig. 4b).
DISCUSSION
The association of smooth muscle cell membrane hyperpolarization with arterial relaxations that are resistant to NOS inhibitors has been interpreted to indicate that another unidentified factor, termed endothelium-derived hyperpolarizing factor, could relax the smooth muscle by that mechanism (2, 3). The chemical measurements of NO release made in the present study of the rabbit carotid artery are, as far as we are aware, the first to be made in a study of endothelium-dependent hyperpolarization. These measurements indicate an alternative interpretation—that NO, the release of which persists in the presence of commonly used NOS inhibitors, is the mediator of both endothelium-dependent relaxation and hyperpolarization. Indeed, both ACh-induced relaxation and hyperpolarization correlated well with the release of NO under control conditions as well as after partial inhibition of the release of NO by l-NAME. The greater inhibition by a very high concentration of l-NNA in combination with l-NAME correlated well with the further inhibition of NO release which occurred. Two techniques to measure NO were used with similar results. Both the instantaneous release of intact NO measured with the porphyrinic microsensor or the release of nitrite that accumulated during exposure of the carotid artery to ACh correlated closely with relaxation and hyperpolarization. The inhibition of NO release by the combination of these inhibitors was limited by their individual inhibitory properties and relative concentrations, because l-NNA had the same effect on NO release as the combination. A 10-fold higher concentration of l-NAME than used in this paper did effectively further inhibit ACh-induced relaxation (7) and NO release (unpublished observations) in the rabbit carotid artery, although even then the effect was incomplete consistent with the competitive nature of the antagonist.
As reported with several other arteries (4, 34, 35) NO was further confirmed as the hyperpolarizing factor released by the endothelium by the finding that the NO donor, SIN-1, which released NO in concentrations in the same range as that released by ACh both relaxed and hyperpolarized cells in the carotid artery. In addition, the present experiments were performed in arteries treated with indomethacin, excluding a potential role of prostacyclin as a hyperpolarizing factor (36). Although other factors may be involved in other arteries or under different experimental conditions, these results in their entirety indicate that NO, rather than a different factor, is the mediator of ACh-induced endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery.
In some blood vessels including the rat (11) and rabbit aorta (7), ACh causes less potent relaxations, and arginine analog inhibitors of NOS effectively block endothelium-dependent relaxation. The release of NO from the rabbit thoracic aorta by ACh is one-fourth of that measured in the rabbit carotid artery (data not shown), providing an explanation for the less potent relaxations and greater sensitivity to NOS inhibitors observed in that blood vessel (7). Thus, the ability of endothelium-dependent vasodilation to persist in the presence of NOS inhibitors may be related more to the amount of NO produced, rather than to the existence of other unidentified mediators. This may be particularly pertinent to smaller vessels in which endothelium-dependent vasodilation persists despite treatment with NOS inhibitors (4, 8–10, 12, 15, 17, 34).
The proposal that other mediators, such as cytochrome P450 metabolites of arachidonic acid (21, 22) or carbon monoxide (23) are alternative endothelium-derived vasodilators rests on experiments in which inhibitors of cytochrome P450 or heme oxygenase block relaxations that persist in the presence of NOS inhibitors. Under similar conditions, cytochrome P450 inhibitors block ACh-induced relaxations of the rabbit carotid artery as well (unpublished observations). The present observation that NO release persists in the presence of the NOS inhibitors and accounts for the smooth muscle response, suggests that cytochrome P450 or heme oxygenase inhibitors may either inhibit the release, scavenge, or prevent the smooth muscle action of NO. Our results indicate that the release of NO must be measured directly before a physiological role for other factors in mediating endothelium-dependent vasodilation can be further postulated.
Complete understanding of the mechanism by which endothelium-derived NO mediates relaxation and hyperpolarization requires further study. Potassium channels could mediate endothelium-dependent smooth muscle cell hyperpolarization (5, 7, 37, 38), and NO has been shown to activate calcium-dependent potassium channels both directly (39) and via cyclic GMP-dependent mechanisms (40, 41). Alternatively, NO could regulate other ionic mechanisms responsible for controlling membrane potential, such as Na+/K+ ATPase (42), which also has been proposed to mediate endothelium-dependent hyperpolarization (43). Whatever the precise explanation for its action on smooth muscle cells, the present studies indicate that NO, released in spite of high concentrations of inhibitors of NOS, is the mediator of endothelium-dependent relaxation and hyperpolarization in the rabbit carotid artery.
Acknowledgments
These studies were supported by National Institutes of Health Grants HL31607, HL55993, HL55854, and HL55397; National Institutes of Health Training Grant HL07429; and a program grant from the Wellcome Trust.
ABBREVIATIONS
- l-NAME
Nω-nitro-l-arginine methyl ester
- l-NNA
Nω-nitro-l-arginine
- SIN-1
3-morpholinosydnonimine hydrochloride
- ACh
acetylcholine
- NO
nitric oxide
- NOS
NO synthase
References
- 1.Feelisch M, te Poel M, Zamora R, Deussen A, Moncada S. Nature (London) 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- 2.Cohen R A, Vanhoutte P M. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 3.Garland C J, Plane F, Kemp B K, Cocks T M. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 4.Garland C J, McPherson G A D. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan C L, Cohen R A. Am J Physiol. 1991;261:H830–H835. doi: 10.1152/ajpheart.1991.261.3.H830. [DOI] [PubMed] [Google Scholar]
- 6.Cowan C L, Cohen R A. J Pharmacol Exp Ther. 1992;260:248–253. [PubMed] [Google Scholar]
- 7.Cowan C L, Palacino J J, Najibi S, Cohen R A. J Pharmacol Exp Ther. 1993;266:1482–1489. [PubMed] [Google Scholar]
- 8.Vallance P, Collier J G, Moncada S. Lancet. 1989;336:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 9.Chowienczyk P J, Cockcroft J R, Ritter J M. Br J Pharmacol. 1993;110:736–738. doi: 10.1111/j.1476-5381.1993.tb13873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugge A, Lopez J A G, Piegors D J, Breese K R, Heistad D D. Am J Physiol. 1991;260:H242–H247. doi: 10.1152/ajpheart.1991.260.1.H242. [DOI] [PubMed] [Google Scholar]
- 11.Rees D D, Palmer R M J, Hodson H F, Moncada S. Br J Pharmacol. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagao T, Illiano S, Vanhoutte P M. Am J Physiol. 1992;263:H1090–H10994. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Suzuki H, Weston A H. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mombouli J V, Illiano S, Nagao T, Scott-Burden T, Vanhoutte P M. Circ Res. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- 15.Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, Fumjishima M. Circ Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- 16.Nagao T, Vanhoutte P M. J Physiol (London) 1992;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeagbo A S O, Triggle C R. J Cardiovasc Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- 18.Nagao T, Vanhoutte P M. Br J Pharmacol. 1992;107:1102–1107. doi: 10.1111/j.1476-5381.1992.tb13414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashima M, Mombouli J V, Taylor A A, Vanhoutte P M. J Clin Invest. 1993;92:2867–2871. doi: 10.1172/JCI116907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzmann S, Kukovetz W R, Windischhofer W, Paschke E, Graier W F. J Cardiovasc Pharmacol. 1994;23:747–756. doi: 10.1097/00005344-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hecker M, Bara A T, Bauersachs J, Busse R. J Physiol (London) 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauersachs J, Hecker M, Busse R. Br J Pharmacol. 1994;113:1548–1553. doi: 10.1111/j.1476-5381.1994.tb17172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakhary R, Gaine S P, Dinerman J L, Ruat M, Flavahan N A, Snyder S H. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archer S L, Hampl V. Biochem Biophys Res Commun. 1992;188:590–596. doi: 10.1016/0006-291x(92)91097-a. [DOI] [PubMed] [Google Scholar]
- 25.Myers P R, Guerra, Harrison D G. J Cardiovasc Pharmacol. 1992;20:392–400. doi: 10.1097/00005344-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Plane F, Najibi S, Cohen R A, Garland C J. Br J Pharmacol. 1996;117:77P. (abstr.). [Google Scholar]
- 27.Najibi S, Cohen R A. Am J Physiol. 1995;269:H805–H811. doi: 10.1152/ajpheart.1995.269.3.H805. [DOI] [PubMed] [Google Scholar]
- 28.Garland C J. J Physiol (London) 1987;392:333–348. doi: 10.1113/jphysiol.1987.sp016783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan S D, Chen X, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt A M. Nature (London) 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 30.Khoury J E, Hickman S E, Thomas C A, Cao L, Silverstein S C, Loike J D. Nature (London) 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 31.Marks G S, McLaughlin B E, Jimmo S L, Poklewska-Koziell M, Brien J F, Nakatsu K. Drug Metab Dispos. 1995;23:1248–1252. [PubMed] [Google Scholar]
- 32.Archer S. FASEB J. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- 33.Mulvany M J, Halpern W. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Waldron G J, Garland C J. Br J Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tare M, Parkington H C, Coleman H A, Neild T O, Dusting G J. Nature (London) 1990;346:69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- 36.Parkington H C, Tare M, Tonta M A, Coleman H A. J Physiol (London) 1993;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor S G, Weston A H. Trends Pharmacol Sci. 1988;9:272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick E V, Cocks T M. Br J Pharmacol. 1994;112:557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolotina V M, Najibi S, Palacino J J, Pagano P J, Cohen R A. Nature (London) 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 40.Archer S L, Huang J M C, Hampl V, Nelson D P, Shultz P J, Weir E K. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson B E, Schubert R, Hescheler J, Nelson M T. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, McArthur C, Grady C, Ruderman N B. Am J Physiol. 1994;266:H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- 43.Feletou M, Vanhoutte P M. Br J Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]