Abstract
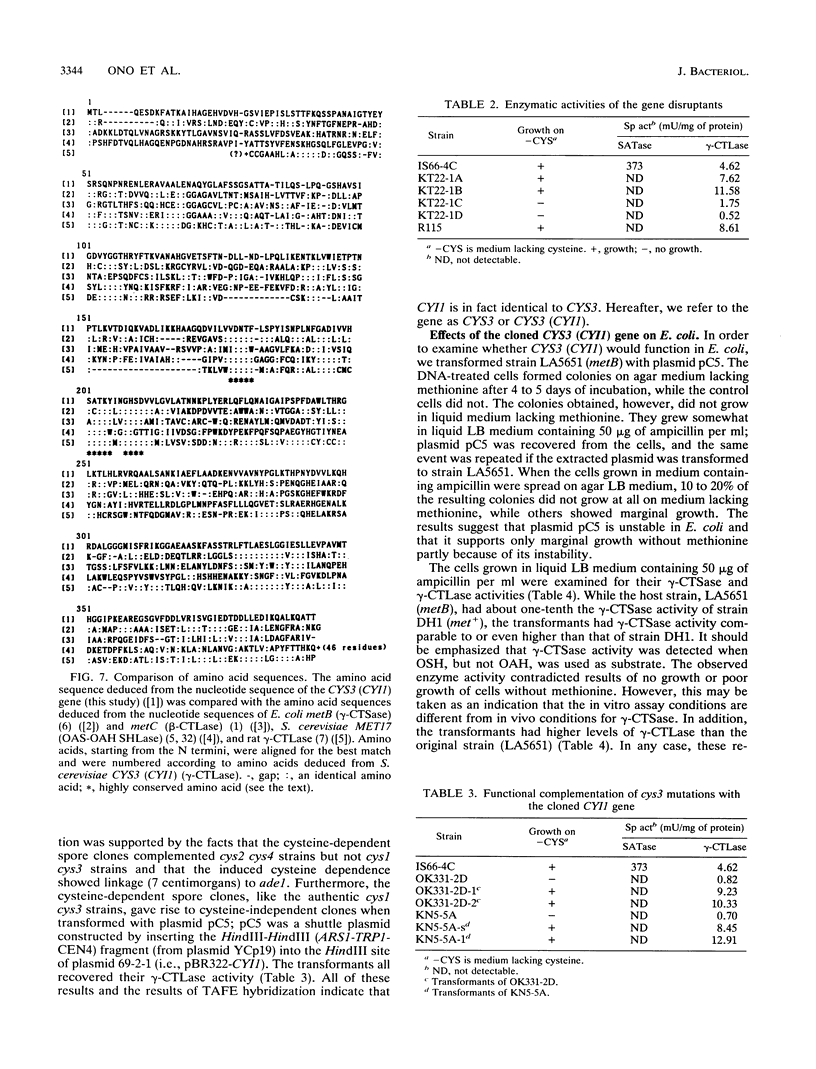
A DNA fragment containing the Saccharomyces cerevisiae CYS3 (CYI1) gene was cloned. The clone had a single open reading frame of 1,182 bp (394 amino acid residues). By comparison of the deduced amino acid sequence with the N-terminal amino acid sequence of cystathionine gamma-lyase, CYS3 (CYI1) was concluded to be the structural gene for this enzyme. In addition, the deduced sequence showed homology with the following enzymes: rat cystathionine gamma-lyase (41%), Escherichia coli cystathionine gamma-synthase (36%), and cystathionine beta-lyase (25%). The N-terminal half of it was homologous (39%) with the N-terminal half of S. cerevisiae O-acetylserine and O-acetylhomoserine sulfhydrylase. The cloned CYS3 (CYI1) gene marginally complemented the E. coli metB mutation (cystathionine gamma-synthase deficiency) and conferred cystathionine gamma-synthase activity as well as cystathionine gamma-lyase activity to E. coli; cystathionine gamma-synthase activity was detected when O-succinylhomoserine but not O-acetylhomoserine was used as substrate. We therefore conclude that S. cerevisiae cystathionine gamma-lyase and E. coli cystathionine gamma-synthase are homologous in both structure and in vitro function and propose that their different in vivo functions are due to the unavailability of O-succinylhomoserine in S. cerevisiae and the scarceness of cystathionine in E. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger C. H., Murray K., Murray N. E. Recognition sequence of a restriction enzyme. Nat New Biol. 1973 Jul 4;244(131):7–10. doi: 10.1038/newbio244007a0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Byrne C. R., Monroe R. S., Ward K. A., Kredich N. M. DNA sequences of the cysK regions of Salmonella typhimurium and Escherichia coli and linkage of the cysK regions to ptsH. J Bacteriol. 1988 Jul;170(7):3150–3157. doi: 10.1128/jb.170.7.3150-3157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea R., Surdin-Kerjan Y., Pure G., Cherest H. Molecular genetics of met 17 and met 25 mutants of Saccharomyces cerevisiae: intragenic complementation between mutations of a single structural gene. Mol Gen Genet. 1987 Apr;207(1):165–170. doi: 10.1007/BF00331505. [DOI] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Erickson P. F., Maxwell I. H., Su L. J., Baumann M., Glode L. M. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990 Jul 15;269(2):335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVIN M., SEGAL A. PURIFICATION AND PROPERTIES OF THE CYSTATHIONINE GAMMA-CLEAVAGE ENZYME OF NEUROSPORA. J Biol Chem. 1964 Jul;239:2220–2227. [PubMed] [Google Scholar]
- Griffith O. W. Mammalian sulfur amino acid metabolism: an overview. Methods Enzymol. 1987;143:366–376. doi: 10.1016/0076-6879(87)43065-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hulanicka M. D., Hallquist S. G., Kredich N. M., Mojica-A T. Regulation of O-acetylserine sulfhydrylase B by L-cysteine in Salmonella typhimurium. J Bacteriol. 1979 Oct;140(1):141–146. doi: 10.1128/jb.140.1.141-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langin T., Faugeron G., Goyon C., Nicolas A., Rossignol J. L. The MET2 gene of Saccharomyces cerevisiae: molecular cloning and nucleotide sequence. Gene. 1986;49(3):283–293. doi: 10.1016/0378-1119(86)90364-1. [DOI] [PubMed] [Google Scholar]
- Martel A., Bouthier de la Tour C., Le Goffic F. Pyridoxal 5'phosphate binding site of Escherichia coli beta cystathionase and cystathionine gamma synthase comparison of their sequences. Biochem Biophys Res Commun. 1987 Sep 15;147(2):565–571. doi: 10.1016/0006-291x(87)90968-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli S., Mevarech M., Ron E. Z. Regulatory region of the metA gene of Escherichia coli K-12. J Bacteriol. 1984 Dec;160(3):1158–1162. doi: 10.1128/jb.160.3.1158-1162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B., Ishino-Arao Y. Inheritance of chromosome length polymorphisms in Saccharomyces cerevisiae. Curr Genet. 1988 Nov;14(5):413–418. doi: 10.1007/BF00521262. [DOI] [PubMed] [Google Scholar]
- Ono B., Naito K., Shirahige Y., Yamamoto M. Regulation of cystathionine gamma-lyase in Saccharomyces cerevisiae. Yeast. 1991 Nov;7(8):843–848. doi: 10.1002/yea.320070809. [DOI] [PubMed] [Google Scholar]
- Ono B., Shirahige Y., Nanjoh A., Andou N., Ohue H., Ishino-Arao Y. Cysteine biosynthesis in Saccharomyces cerevisiae: mutation that confers cystathionine beta-synthase deficiency. J Bacteriol. 1988 Dec;170(12):5883–5889. doi: 10.1128/jb.170.12.5883-5889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B., Suruga T., Yamamoto M., Yamamoto S., Murata K., Kimura A., Shinoda S., Ohmori S. Cystathionine accumulation in Saccharomyces cerevisiae. J Bacteriol. 1984 Jun;158(3):860–865. doi: 10.1128/jb.158.3.860-865.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Role of cysteine and-or homocysteine as regulatory effectors. Mol Gen Genet. 1974;132(4):307–320. doi: 10.1007/BF00268571. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsoda S., Cherest H., Surdin-Kerjan Y. The expression of the MET25 gene of Saccharomyces cerevisiae is regulated transcriptionally. Mol Gen Genet. 1985;200(3):407–414. doi: 10.1007/BF00425724. [DOI] [PubMed] [Google Scholar]
- Sirko A., Hryniewicz M., Hulanicka D., Böck A. Sulfate and thiosulfate transport in Escherichia coli K-12: nucleotide sequence and expression of the cysTWAM gene cluster. J Bacteriol. 1990 Jun;172(6):3351–3357. doi: 10.1128/jb.172.6.3351-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K. Microbial sulfur amino acids: an overview. Methods Enzymol. 1987;143:453–459. doi: 10.1016/0076-6879(87)43080-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Mann C., Davis R. W. Centromeric DNA from Saccharomyces cerevisiae. J Mol Biol. 1982 Jun 25;158(2):157–190. doi: 10.1016/0022-2836(82)90427-2. [DOI] [PubMed] [Google Scholar]
- Uren J. R. Cystathionine beta-lyase from Escherichia coli. Methods Enzymol. 1987;143:483–486. doi: 10.1016/0076-6879(87)43086-3. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in yeast. J Biochem. 1974 Jun;75(6):1221–1229. doi: 10.1093/oxfordjournals.jbchem.a130505. [DOI] [PubMed] [Google Scholar]
- Yamane A., Nakagami S., Miyoshi K. Applications of universal probe on DNA hybridization. Nucleic Acids Symp Ser. 1988;(19):93–95. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]