Abstract
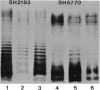
Lipopolysaccharides (LPS) from the type strains of the anaerobic beer spoilage bacteria Pectinatus cerevisiiphilus and P. frisingensis were extracted with the 5:5:8 volume ratio modification of the phenolchloroform-petroleum ether method (H. Brade and C. Galanos, Eur. J. Biochem. 122:233-237, 1982). Sequential precipitations of LPS with water and acetone from the phenol phase yielded LPS which differed in that water-precipitable material (LPS-H2O; 0.1 to 0.4% of the dry weight of the cells) was rough-type LPS, whereas acetone-precipitable material (LPS-Ac; 4.6 to 5.8% of the dry weight) contained both rough-type LPS and high-molecular-weight material resembling smooth LPS. The LPS were chemically characterized, and they contained D-glucosamine, 4-amino-4-deoxy-L-arabinose, 3-deoxy-D-manno-2-octulosonic acid, D-fucose, D-galactose, D-glucose, D-mannose, and phosphate. D-Fucose was present mostly in LPS-Ac, suggesting that it is a constituent of the O antigen. The major fatty acids were ester- and amide-linked (R)-3-hydroxytridecanoic and ester-linked undecanoic acids, with minor amounts of ester-linked tridecanoic and (R)-3-hydroxyundecanoic acids. The chemical compositions of LPS-H2O and LPS-Ac suggested that they differ not only in their smooth or rough nature but also in the structure of their core regions. This may explain their different precipitabilities from the extraction mixture. The extraction method was also shown to be applicable to the isolation of smooth-type LPS from Salmonella enterica serovar Typhimurium. Extraction of two Typhimurium strains carrying chemically different O antigens resulted in high yields (8% of the dry weight) of LPS. Strain SH2183, which contains the relatively hydrophobic O-4,5,12 antigen yielded almost exclusively LPS-Ac, whereas the LPS of strain SH5770, which has a hydrophilic O-6,7 antigen, was exclusively LPS-H2O. No fractionation to smooth and rough LPS occurred with the Typhimurium strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bordet C., Bruneteau M., Michel G. Lipopolysaccharides d'une souche à virulence atténuée de Yersinia pestis. Eur J Biochem. 1977 Oct 3;79(2):443–449. doi: 10.1111/j.1432-1033.1977.tb11826.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C. Isolation, purification, and chemical analysis of the lipopolysaccharide and lipid A of Acinetobacter calcoaceticus NCTC 10305. Eur J Biochem. 1982 Feb;122(2):233–237. doi: 10.1111/j.1432-1033.1982.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Fischer W. Purification and fractionation of lipopolysaccharide from gram-negative bacteria by hydrophobic interaction chromatography. Eur J Biochem. 1990 Dec 12;194(2):655–661. doi: 10.1111/j.1432-1033.1990.tb15665.x. [DOI] [PubMed] [Google Scholar]
- Flowers H. M. Chemistry and biochemistry of D- and L-fucose. Adv Carbohydr Chem Biochem. 1981;39:279–345. doi: 10.1016/s0065-2318(08)60208-5. [DOI] [PubMed] [Google Scholar]
- Galanos C., Jiao B. H., Komuro T., Freudenberg M. A., Lüderitz O. Large-scale fractionation of S-form lipopolysaccharide from Salmonella abortus equi. Chemical and serological characterization of the fractions. J Chromatogr. 1988 May 25;440:397–404. doi: 10.1016/s0021-9673(00)94543-6. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Haikara A., Penttilä L., Enari T. M., Lounatmaa K. Microbiological, biochemical, and electron microscopic characterization of a pectinatus strain. Appl Environ Microbiol. 1981 Feb;41(2):511–517. doi: 10.1128/aem.41.2.511-517.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S., Reitschel E. T. The chemical structure of the lipid A component of lipopolysaccharides from Chromobacterium violaceum NCTC 9694. Eur J Biochem. 1977 May 2;75(1):23–34. doi: 10.1111/j.1432-1033.1977.tb11500.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Lindner B., Brade H., Altmann K., Lindberg A. A., Rietschel E. T., Zähringer U. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur J Biochem. 1988 Nov 15;177(3):483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao B. H., Freudenberg M., Galanos C. Characterization of the lipid A component of genuine smooth-form lipopolysaccharide. Eur J Biochem. 1989 Apr 1;180(3):515–518. doi: 10.1111/j.1432-1033.1989.tb14676.x. [DOI] [PubMed] [Google Scholar]
- Knirel' Iu A., Shashkov A. S., Soldatkina M. A., Paramonov N. A., Zakharova I. Ia. Antigennye polisakharidy bakterii. 32. Struktura O-spetsificheskikh polisakharidnykh tsepei lipopolisakharidov Pseudomonas cepacia serotipov B i E, soderzhashchikh D-fukozu. Bioorg Khim. 1988 Sep;14(9):1208–1213. [PubMed] [Google Scholar]
- Knirel' Iu A., Zdorovenko G. M., Shashkov A. S., Gubanova N. Ia, Iakovleva L. M. Antigennye polisakharidy bakterii. 27. Stroenie O-spetsificheskoi polisakharidnoi tsepi lipopolisakharidov Pseudomonas syringae, patovary atrofaciens 2399, phaesolicola 120a, i Pseudomonas holci 8299, otnosiashchikhsia k serogruppe VI. Bioorg Khim. 1988 Jan;14(1):92–99. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Moran A. P., Helander I. M., Kosunen T. U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Rietschel E. T., Kosunen T. U., Zähringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J Bacteriol. 1991 Jan;173(2):618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Ray T. C., Smith A. R., Wait R., Hignett R. C. Structure of the sidechain of lipopolysaccharide from Erwinia amylovora T. Eur J Biochem. 1987 Dec 30;170(1-2):357–361. doi: 10.1111/j.1432-1033.1987.tb13707.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T. Absolute configuration of 3-hydroxy fatty acids present in lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 May 1;64(2):423–428. doi: 10.1111/j.1432-1033.1976.tb10318.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Schleifer K. H., Leuteritz M., Weiss N., Ludwig W., Kirchhof G., Seidel-Rüfer H. Taxonomic study of anaerobic, gram-negative, rod-shaped bacteria from breweries: emended description of Pectinatus cerevisiiphilus and description of Pectinatus frisingensis sp. nov., Selenomonas lacticifex sp. nov., Zymophilus raffinosivorans gen. nov., sp. nov., and Zymophilus paucivorans sp. nov. Int J Syst Bacteriol. 1990 Jan;40(1):19–27. doi: 10.1099/00207713-40-1-19. [DOI] [PubMed] [Google Scholar]
- Sidorczyk Z., Zähringer U., Rietschel E. T. Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re-mutant. Eur J Biochem. 1983 Dec 1;137(1-2):15–22. doi: 10.1111/j.1432-1033.1983.tb07789.x. [DOI] [PubMed] [Google Scholar]
- Smith A. R., Zamze S. E., Munro S. M., Carter K. J., Hignett R. C. Structure of the sidechain of lipopolysaccharide from Pseudomonas syringae pv. morsprunorum C28. Eur J Biochem. 1985 May 15;149(1):73–78. doi: 10.1111/j.1432-1033.1985.tb08895.x. [DOI] [PubMed] [Google Scholar]
- Tharanathan R. N., Weckesser J., Mayer H. Structural studies on the D-arabinose-containing lipid A from Rhodospirillum tenue 2761. Eur J Biochem. 1978 Mar 15;84(2):385–394. doi: 10.1111/j.1432-1033.1978.tb12179.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Jensen M., Helander I., Nurminen M., Rietschel E. T., Mäkelä P. H. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 1981 Jun 29;129(1):145–149. doi: 10.1016/0014-5793(81)80777-6. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V. Role of phagocytosis in mouse virulence of Salmonella typhimurium recombinants with O antigen 6,7 or 4,12. Infect Immun. 1977 Dec;18(3):574–582. doi: 10.1128/iai.18.3.574-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]