Abstract
Estrogen deficiency induced bone loss is associated with increased bone turnover in rats and humans. The respective roles of increased bone turnover and altered balance between bone formation and bone resorption in mediating estrogen deficiency-induced cancellous bone loss was investigated in ovariectomized rats. Ovariectomy resulted in increased bone turnover in the distal femur. However, cancellous bone was preferentially lost in the metaphysis, a site that normally experiences low strain energy. No bone loss was observed in the epiphysis, a site experiencing higher strain energy. The role of mechanical strain in maintaining bone balance was investigated by altering the strain history. Mechanical strain was increased and decreased in long bones of ovariectomized rats by treadmill exercise and functional unloading, respectively. Functional unloading was achieved during orbital spaceflight and following unilateral sciatic neurotomy. Increasing mechanical loading reduced bone loss in the metaphysis. In contrast, decreasing loading accentuated bone loss in the metaphysis and resulted in bone loss in the epiphysis. Finally, administration of estrogen to ovariectomized rats reduced bone loss in the unloaded and prevented loss in the loaded limb following unilateral sciatic neurotomy in part by reducing indices of bone turnover. These results suggest that estrogen regulates the rate of bone turnover, but the overall balance between bone formation and bone resorption is influenced by prevailing levels of mechanical strain.
Ovarian hormone deficiency is the most important risk factor for postmenopausal osteoporosis (1, 2). Bone loss also occurs in premenopausal women following ovariectomy (OVX) (3) or treatment with gonadotrophin-releasing hormone agonists (4). Estrogen replacement therapy prevents bone loss in postmenopausal and ovariectomized women, suggesting that 17β-estradiol is the gonadal hormone that is essential for normal bone balance.
The mechanism for the skeletal effects of estrogen are incompletely understood but have been the subject of intense study in laboratory animal models (5). The rat has proven to be especially useful. OVX and gonadotrophin-releasing hormone agonists result in bone loss in rats, and these changes are prevented by estrogen treatment (6–9). These observations suggest similar skeletal mechanisms of action of estrogen in rats and humans. Furthermore, the skeletal changes in rats in response to partial estrogen agonists have accurately predicted the differential responses of pre- and postmenopausal women to tamoxifen treatment (10, 11).
The bone loss in postmenopausal women and ovariectomized women and rats is associated with elevated bone turnover (6, 12–14). However, the bone loss is not uniform; cancellous bone is at a greater risk than cortical bone (7, 14, 15). In addition, there is site specificity in the loss of cancellous bone. For example, cancellous bone is lost more rapidly from the proximal tibial metaphysis than from vertebral bodies (16). Also, bone is preferentially lost from the proximal tibia; cancellous bone is not lost from the distal metaphysis (17).
Skeletal unweighting, whether due to spaceflight, prolonged bedrest, paralysis, localized stress shielding following arthroplasty, or cast immobilization leads to bone loss in humans and laboratory animal models (18–22). Profound, direct effects of mechanical loading have been established by locally loading skeletal tissues in vivo (23, 24) and bone cells in culture (25). The effects of mechanical loading are additionally influenced by systemic factors, including sex steroids (26).
The basis for differential bone loss in ovariectomized rats is important to understanding the physiological actions of estrogen on bone metabolism and may be relevant to the prevention of postmenopausal osteoporosis. With this possibility in mind, we investigated the interrelationship between bone loss, bone turnover, and mechanical loading in the ovariectomized rat model. The results of these studies indicate that the overall rate of cancellous bone turnover is regulated by estrogen but that the balance between bone formation and bone resorption is modulated by an additional factor, mechanical loading.
MATERIALS AND METHODS
All animal studies were approved by the appropriate institutional animal welfare committees at the Mayo Foundation, the Pennsylvania State University, the University of Florida, the Charleston Veterans Administration Medical Center, and the National Aeronautics and Space Administration.
Experiment 1.
Twenty female Sprague–Dawley rats (n = 10/group) were OVX or sham-operated at 3 months of age. The animals were killed 11.5 months later and the femora were excised and immediately frozen in liquid N2 for future RNA isolation and histology. OVX was confirmed at the time of sacrifice in this and each subsequent experiment by the presence of atrophic uterine horns and lack of ovarian tissue.
The frozen left femora from six animals were fixed in unlabeled 70% ethanol for a minimum of 2 days, dehydrated in an ascending series of increasing concentrations of ethanol, embedded undemineralized in a mixture of methyl methacrylate/2-hydroxyethyl-methacrylate (12.5:1), and sectioned at an indicated thickness of 5 μm. Histomorphometric procedures on the left femur were carried out using a SMI-Microcomp semiautomatic image analysis system (Southern Micro Instruments, Atlanta) consisting of a Compaq computer with Microcomp software interphased with a microscope and image analysis system. In this system, a high resolution video camera mounted on an Olympus BH-2 microscope displays the image of the specimen on a color monitor. The movement of a pen on a graphics tablet superimposes a tracing of the specimen on the video screen. By this method, the region of interest is traced, and the line length and area bounded by the tracing are calculated. The sampling sites in the distal femur consisted of the epiphysis (12 mm2/bone) and the area immediately distal to the growth plate extending 7 mm to encompass the entire metaphysis (35 mm2).
Declomycin was administered 19 and 18 days prior to sacrifice and calcein was administered 8 and 7 days before sacrifice. Labeled perimeter, mineral apposition rate, and bone formation rate were determined as described (27). Eroded perimeter was determined as the scalloped cancellous bone perimeter. Trabecular thickness (Tb.Th) was calculated as 2 ÷ B.Pm/T.Pm. Trabecular number (Tb.N) was calculated as B.Ar/Tb.Th.
The frozen left femora from four animals per group were cut at the distal end of the third trochanter and the distal portion was individually homogenized in guanidine isothiocyanate using a Spex Freezer Mill (Edison, NJ). Cancellous bone from the tibial metaphysis was extracted as described (28) and individual samples were powdered using the Freezer Mill. Total cellular RNA was extracted and isolated using a modified organic solvent method (29) and the yields were determined spectrophotometrically at 260 nm. Ten micrograms of each sample were denatured by incubation at 52°C in a solution of 1 M glyoxal/50% dimethyl sulfoxide in 0.1 M NaH2PO4 solution, then separated electrophoretically in a 1% agarose gel. The amounts of RNA loaded and transferred were assessed by ethidium bromide staining of the gels and hybridization of the filters with a 32P-labeled cDNA for glyceraldehyde-3-phosphate dehydrogenase (GAP).
The RNA was transferred overnight via capillary action in 20× sodium saline citrate (SSC) buffer to an Amersham Hybond nylon membrane and cross-linked with a Stratagene UV Stratalinker 1800 before hybridization. The filters were prehybridized for 6 h at 45°C in a buffer containing 50% deionized formamide, 10% dextran sulfate, 5× SSC (1× SSC = 0.15 M NaCl/0.015 M sodium citrate, pH 7.0), 600 mg/ml heat denatured single-strand salmon sperm DNA, and 2× Denhardt’s solution. Hybridization was carried out for 18–24 h in a buffer containing the above ingredients in addition to a minimum of 106 cpm/ml 32P-labeled cDNA for osteocalcin (OC), prepro α2 (I) chain of type I collagen (collagen), or GAP. GAP was used to correct for unequal loading of RNA in the agarose gel. cDNA probes were labeled by random sequence hexanucleotide primer extension using the Megaprime DNA labeling kit from Amersham. The filters were washed for 30 minutes at 45°C in 2× SSC and for 15–60 min in 0.1× SSC at 45°C. The mRNA bands on the Northern blots were quantitated by densitometric scanning using a Molecular Dynamics PhosphorImager.
The cDNA probes used were rat OC, a gift from S. Rossi-Langer (Genetics Institute, Cambridge, MA) (30); human collagen, a gift from H. Hivanlem and G. Tromp (Jefferson Medical College, Philadelphia) (31); and rat GAP, a gift from P. Forti (Laboratorie de Biologie Moleculaire, Montpellier, France) (32).
The strain energy density distribution in the distal femur was predicted by a two-dimensional finite element model. The bony geometry was determined from the x-ray film of a specimen. The material properties used were: Elastic modulus 7 GPa, Poisson’s ratio 0.3 for cortical bone, and elastic modulus 0.9 GPa, Poisson’s ratio = 0.3 for cancellous bone (33). The joint force was assumed as 1.64 N, which was about one-third of an average rat body weight (500 g). The three-dimensional effects on this two-dimensional model were considered by including the side plate (34). The analysis was performed on Silicon Graphics Computer (IRIS 4D/420 VGX) with finite element code abaqus (Hibbitt, Karlsson & Sorenson, Pawtucket, RI).
The microtomographic system designed is based on a micro-CT scanner developed by Flannery et al. (35). Methylmethacrylate embedded OVX and ovary-intact specimens from experiments 1 and 4 were scanned simultaneously to ensure equal exposure. The scanner scans a volume of ≈15 cm3. The three-dimensional images are generally reconstructed with 20-μm on-a-side cubic voxels (three-dimensional picture elements). In some cases the projected views are magnified during the scan to generate three-dimensional images of rat femur with 2.6-μm voxel resolution.
Experiment 2.
Fisher 344 rats from Taconic Farms were ovariectomized at 10–11 weeks of age and divided into three groups (n = 6/group). Older animals could not be used because of weight limitations. Fisher 344 rats were used because the slow growth rate of this strain minimizes growth-related changes. This was important because OVX increases the rates of longitudinal bone growth and resorption of calcified growth plate cartilage. The baseline animals were killed 2 weeks following OVX and immediately following launch of the space shuttle Columbia (Mission STS-62). The flight and ground control animals were maintained in AEM (animal enclosure module) hardware for the duration of the 14-day spaceflight as described (36). The flight animals were killed 4–7 h following the flight whereas the ground control experiment was started and stopped 2 days later than the flight animals so that orbiter temperature and humidity data could be retrieved and used to emulate the animal environment aboard the shuttle.
Experiment 3.
Seven-week-old Sprague–Dawley rats from Holtzmann (Madison, WI) were ovariectomized or sham-operated and 1 week later treadmill exercise was initiated. The rats were exercised 5 times a week for 6 weeks with the following schedule: speed was increased from 15 m/min and 1% grade for 10 min by 2 m/min (rate), 10 min/week (time), and 1% every other day (grade) to a maximum of 25 m/min and 5% grade for 30 min as described (37, 38).
Experiment 4.
Seventy-six-week-old ovariectomized and 10 ovary-intact Fisher 344 rats were obtained from Taconic Farms. Groups of 10 rats were killed 0 (ovary-intact baseline controls), 2, 4, and 7.5 weeks following OVX. At 7.5 weeks the remaining 40 animals underwent unilateral (right limb) sciatic neurotomy (USN) (39). Half of the animals (+E) were implanted with controlled release pellets containing 17β-estradiol (estradiol) (Innovative Research of America). The remaining rats were implanted with pellets containing the cellulose carrier only. These pellets were designed to release a total of 0.2 mg estradiol at a continuous rate for 6 weeks. Groups of estradiol and carrier treated rats were killed 2 and 6 weeks following USN. Cancellous bone area was determined for the proximal tibial epiphysis and metaphysis of the weight bearing and unloaded limbs.
Dynamic bone measurements were performed 2 weeks following USN to evaluate the respective effects of estrogen and weight bearing on cancellous bone turnover. Calcein (20 mg/kg) and tetracycline (20 mg/kg) were administered to the rats on the day of surgery and 13 days following USN, respectively. Baseline controls were killed 1 day following USN. The remaining rats were killed 14 days following surgery. The mineral apposition rate was determined as in experiment 1. The resorption of the cancellous bone was determined as described (27). Briefly, calcein perimeter was measured in the tibial metaphysis at a growth adjusted site and compared with the baseline measurements. Resorption (R) was calculated as R = 100% − % baseline.
RESULTS
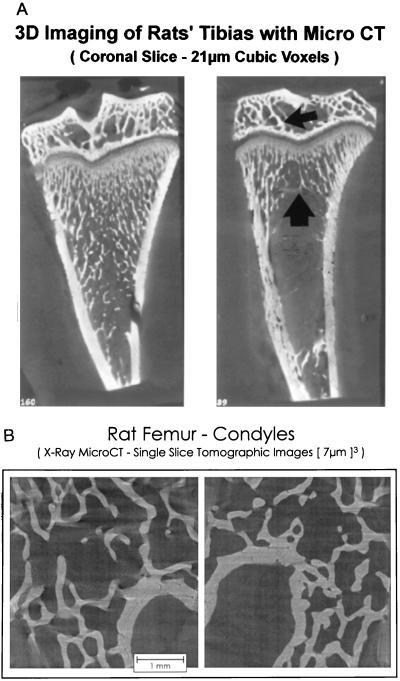
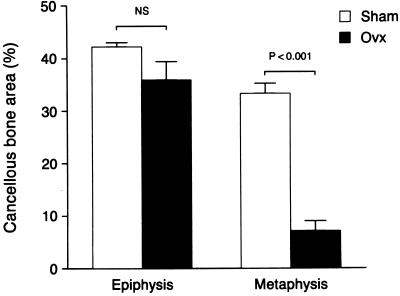
Microtomographic images of proximal tibiae and distal femur epiphyses are shown in Fig. 1 to illustrate the regions sampled and qualitative changes which follow OVX. The quantitative effects of long-term ovarian hormone deficiency on cancellous bone volume in the distal femur are shown in Fig. 2. OVX had no significant effect on cancellous bone area in the epiphysis whereas there was dramatic bone loss in the metaphysis. There were notable changes in cancellous architecture in the metaphysis (Table 1). Trabecular number was greatly diminished in the ovariectomized rats. Interestingly, the remaining few trabeculae were located at the cortical margins and appeared to be as robust as the more numerous trabeculae in the ovary-intact animals. This perception was verified by calculation of mean trabecular thickness that was not influenced by OVX. In contrast, following OVX there were small, nonsignificant decreases in epiphyseal trabecular number and thickness.
Figure 1.
(A) Comparative effects of 1 month of gonadal insufficiency following OVX on cancellous bone volume in the proximal tibia. Representative microtomographic images from an ovary-intact (Left) and OVX (Right) rat showing greatly decreased cancellous bone in the metaphysis (large arrow) but little change in the epiphysis (small arrow) following OVX. (B) Comparative effects of 11.5 months of gonadal insufficiency following OVX on cancellous bone volume in the distal femur. Representative microtomographic images from an ovary-intact (Left) and OVX (Right) showing little change in cancellous bone in the epiphysis even though there was dramatic loss from the metaphysis (Fig. 2).
Figure 2.
Comparative effects of 11.5 months of gonadal insufficiency following OVX on cancellous bone volume in the distal femur. Quantitative histomorphometry revealed that the metaphysis undergoes a significant decrease in cancellous bone area whereas there was no change in the epiphysis. Values are mean ± SE; n = 6.
Table 1.
Comparative effects of 11.5 months of gonadal insufficiency following OVX on cancellous architecture in the distal femur (experiment 1)
| Group | Trabecular number, μm−1 | Trabecular thickness, mm |
|---|---|---|
| Metaphysis | ||
| Control | 4.92 ± 0.31 | 0.068 ± 0.003 |
| OVX | 1.15 ± 0.14 | 0.062 ± 0.010 |
| P value | <0.001 | NS |
| Epiphysis | ||
| Control | 3.17 ± 0.18 | 0.135 ± 0.009 |
| OVX | 2.66 ± 0.16 | 0.133 ± 0.005 |
| P value | NS | NS |
Values are mean ± SE (n = 6). NS = P > 0.05.
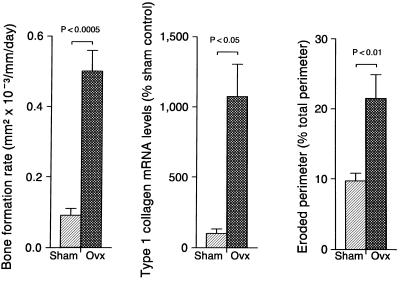
The effects of OVX on selected indices of cancellous bone turnover in the epiphysis are shown in Fig. 3. OVX resulted in dramatic increases in the calculated bone formation rate, steady-state mRNA levels for the bone matrix protein type 1 collagen and eroded bone perimeter. The principal reason for the increase in bone formation in ovariectomized rats was a 370% increase (P < 0.0001) in labeled perimeter from 9.7 ± 1.1 to 44.1 ± 22%; mean ± SE. Secondarily, there was also a 21% nonsignificant (P > 0.05) increase in the mineral apposition rate from 0.91 ± 0.08 to 1.11 ± 0.04 μm/day. The increase in message levels in the epiphysis was not limited to type 1 collagen; OVX resulted in a 216% increase (P < 0.05) in steady-state mRNA levels for OC from 100 ± 35 to 316 ± 101 (% of intact control).
Figure 3.
Data for 11.5 months of gonadal insufficiency result in greatly elevated indices of bone turnover in the distal femur epiphysis. The results demonstrate that increased bone turnover does not in-and-of-itself result in bone loss. Values are mean ± SE; n = 4 for steady-state mRNA levels and n = 6 for histomorphometric values.
There was evidence for a similar increase in bone turnover in the metaphysis of OVX rats. The labeled bone perimeter in the few remaining trabeculae was increased 476% (P < 0.001) while the mineral apposition rate was increased 45% (P < 0.05). In addition, tissue mRNA levels for type 1 collagen and OC were increased significantly compared with the intact control (P < 0.05) by 265% (from 100 ± 65 to 365 ± 85%) and 135% (from 100 ± 33% to 235 ± 104%), respectively.
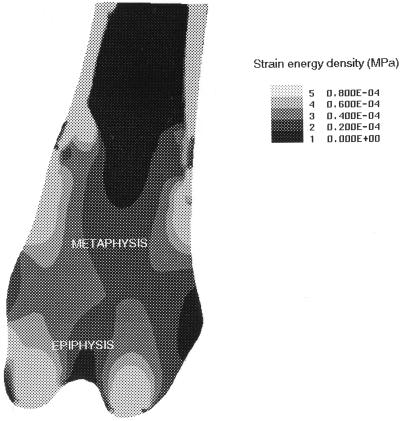
The strain energy distribution is shown for the distal femur in Fig. 4. The central region of the cancellous metaphysis is under relatively low strain energy whereas the cortical margins, epiphysis, and cortical bone experience higher strain energies.
Figure 4.
The strain energy distribution within the distal femur predicted by a two-dimensional finite element model. Lighter areas represent high strain energy whereas darker areas represent low strain energy. The cancellous bone loss which follows OVX occurs predominantly at sites subjected to low strain energies.
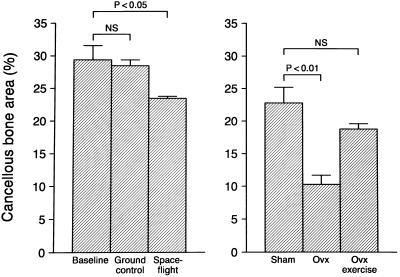
The near weightlessness of orbital spaceflight resulted in cancellous bone loss in the epiphysis (Fig. 5) as well as the metaphysis (data not shown). In contrast, treadmill exercise prevented cancellous bone loss from the metaphysis of ovariectomized rats (Fig. 5).
Figure 5.
Decreased weight bearing (spaceflight) results in cancellous bone loss from the proximal tibial epiphysis of OVX rats. Increased weight bearing (treadmill exercise) antagonizes cancellous bone loss at the proximal tibial metaphysis. Values are mean ± SE; n = 8–10; P < 0.05 compared with either baseline or sham control.
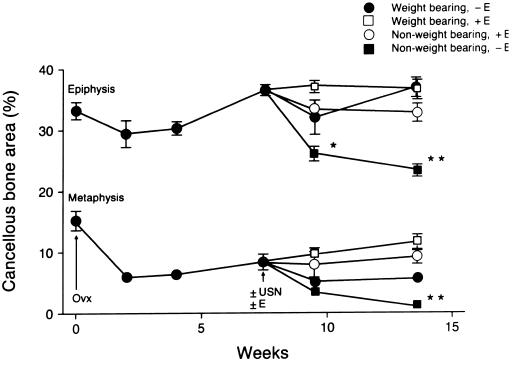
The time course for bone loss in ovariectomized growing rats is shown in Fig. 6. Significant bone loss occurred within the metaphysis within 2 weeks of OVX whereas no change occurred in the epiphysis. USN resulted in bone loss in the epiphysis of OVX rats and further bone loss in the metaphysis (Fig. 6). Pharmacological replacement with estrogen in ovariectomized USN rats prevented the bone loss in the epiphysis and prevented the further bone loss in the metaphysis. Disuse depressed the mineral apposition rate in the cancellous metaphysis in OVX rats while estrogen treatment decreased resorption of a pretreatment fluorochrome label and mineral apposition rate (Table 2).
Figure 6.
Comparative effects of weight bearing and estrogen on cancellous bone volume in OVX rats. Rats were ovariectomized 7.5 weeks prior to USN to establish bone loss in the tibial metaphysis. Following OVX, there was no significant change in bone area in the epiphysis whereas bone area was reduced in the metaphysis (P < 0.05) at all time points. In contrast, USN resulted in bone loss in the epiphysis and further bone loss in the metaphysis. Half of the rats were implanted with subcutaneous implants designed to deliver 17β-estradiol at a constant rate totaling 0.2 mg during a 6-week interval (+E). Estrogen treatment prevented bone loss following USN in the epiphysis and prevented further bone loss in the metaphysis. Values are mean ± SE; n = 10. ∗, P < 0.05; ∗∗, P < 0.01 compared with baseline controls (time 0). Figure data points without error bars are larger than ± SE.
Table 2.
Effects of USN and estradiol on dynamic bone measurements in proximal tibial metaphysis of OVX rats
| Measurement | Unloaded
|
Loaded
|
Two Factor Analysis
|
||||
|---|---|---|---|---|---|---|---|
| − Estrogen | + Estrogen | − Estrogen | + Estrogen | Estrogen | Loading | Interaction | |
| Resorption of calcein label, % | 66.4 ± 3.8 | 41.5 ± 9.7 | 59.0 ± 7.4 | 38.9 ± 7.4 | <.009 | NS | NS |
| Mineral apposition rate | 1.42 ± 0.12 | 1.19 ± 0.09 | 1.85 ± 0.08 | 1.33 ± 0.08 | <.007 | <.05 | NS |
NS, not significant.
DISCUSSION
The distal metaphysis of the femur of rats is exquisitely sensitive to estrogen deprivation; OVX results in a rapid and profound cancellous osteopenia that is prevented by estrogen replacement (5). We have noticed that the trabecular architecture of the adjacent epiphysis appears to be comparatively resistant to bone loss following OVX. To verify this impression, we compared the effects of long-term estrogen deficiency on the two cancellous bone compartments in the distal femur. Our data clearly show that 11.5 months following OVX there was no significant change in mean cancellous bone area in the epiphysis, whereas the metaphysis became severely osteopenic. The difference between the two sites is especially striking because most of the bone loss in the metaphysis typically occurs within 2 months following OVX (40).
To confirm that the epiphysis is an estrogen target tissue, we evaluated the effects of OVX on bone turnover in this tissue. The results revealed that OVX results in pronounced increases in indices of bone formation (calculated bone formation rate and steady-state mRNA levels for the bone matrix proteins type I collagen and OC) and bone resorption (eroded perimeter). The changes were comparable or greater than those measured in the metaphysis in this and previous short- and long-term studies (6, 27, 28). Our results provide compelling evidence that the epiphysis is highly responsive to gonadal hormone deficiency, implying that increased bone turnover resulting from estrogen deficiency does not necessarily result in bone loss. This conclusion is supported by the work of Ke et al. (17) who demonstrated that following OVX cancellous bone is lost more rapidly from the rat proximal tibial metaphysis than from the distal metaphysis.
Mechanical strain is critical to establish and maintain normal bone architecture (26, 41–45). A change in bone mass will initially result in a reciprocal change in bone mechanical strain. Also, changes in either body weight or physical activity will result in proportional changes in bone strain. In turn, altered strain levels influence bone cell numbers and activities that result in changes in bone mass and architecture that restore former levels of bone mechanical strain. The bone’s mechanical set point or “mechanostat” represents the range in strain values over which the bone can be subjected prior to a compensatory response in altered bone formation or resorption (46). This counter regulatory mechanism is evident in many species (47, 48) and serves to maintain strain levels in bone at ≈1500 micro-strain (49–51). It is therefore somewhat surprising that cancellous bone loss following OVX, although most rapid during the early stages of estrogen deficiency, continues over a prolonged interval. The bone loss occurs without a well-defined compensatory response to the increasing strain energies experienced by the surviving trabeculae. One possible explanation for this defect in bone homeostasis is that estrogen deficiency increases the mechanostat threshold at which bone cells respond to mechanical strain (41, 52). If this hypothesis is correct, trabeculae initially subjected to the lowest mechanical strain are at the greatest risk to be resorbed in the absence of estrogen.
To evaluate this hypothesis, finite element analysis was used to model the strain energy distribution pattern in the distal femur. The results demonstrate an excellent correspondence between strain energy magnitude and resistance to estrogen deficiency-induced bone loss. The central region of the cancellous metaphysis was under low strain energy in ovary-intact rats and is the initial region resorbed following OVX. The principal mechanism for the bone loss was removal of trabecular plates; gradual trabecular thinning was not observed. This finding is in good agreement with the observations of Dempster et al. (53). A similar pattern of bone loss was observed in the metaphysis during longitudinal bone growth (54). The destruction of the trabeculae most distal to the growth plate during growth may be another example of strain energy driven bone modeling. In contrast, the cortical margins of the metaphysis and epiphysis are under greater strain energy and are preserved even following long-term OVX. Interestingly, the few remaining trabeculae in the metaphysis 11.5 months following OVX are as robust as the average trabeculae in the ovary-intact controls.
To further test the strain hypothesis, experiments to increase and decrease strain were performed. Strain energy was decreased and increased in ovariectomized rats by subjecting the animals to the near weightlessness of orbital spaceflight and treadmill exercise, respectively. As expected, reducing mechanical strain energy resulted in cancellous bone loss from the epiphysis of estrogen-deficient rats, whereas increasing weight bearing reduced loss of cancellous bone from the metaphysis. The latter findings are in agreement with previous studies reporting that exercise attenuates OVX-induced bone loss (55, 56). We have extended these findings to evaluate the interaction between weight bearing and estrogen. In these latter studies, USN was used to reduce mechanical strain. USN of ovariectomized rats resulted in rapid loss of cancellous bone from the epiphysis of the unloaded limb, but no loss from the weight-bearing limb. Also, USN resulted in cancellous bone loss in the metaphysis of OVX rats in excess of that resulting from ovarian hormone deficiency. We interpret these data as evidence that estrogen deficiency leads to increased bone turnover and net bone resorption until the strain levels experienced by the surviving trabeculae exceed the new threshold. The rate of bone turnover remains elevated in OVX rats with established osteopenia but the balance between bone formation and resorption must become reestablished to prevent further bone loss. Mechanical unloading due to USN reduces strain in estrogen-deficient rats and results in additional bone loss. On the other hand, administration of estrogen might be expected to counteract bone loss in animals with reduced mechanical unloading by nature of the hormone’s potent inhibitory action on bone turnover (5, 8, 27). Indeed, pharmacological intervention of OVX/USN rats with estrogen treatment prevented bone loss in the epiphysis and halted bone loss in the metaphysis. Furthermore, estrogen reduced indices of bone turnover in unweighted as well as weight-bearing limbs of OVX rats.
The independent regulation of bone volume by mechanical loading and estrogen may be relevant to calcium homeostasis during reproduction. Estrogen is essential for maintenance of medullary bone; medullary bone functions as a mineral reserve for eggshell calcification in birds, and is not under mechanical loading (5). In mammals, strain energy levels may dictate the pattern of bone loss during lactation (5, 57, 58).
In summary, our results demonstrate that estrogen deficiency results in increased levels of bone turnover at sites that lose bone as well as sites that resist bone loss. In contrast, mechanical strain modulates the balance between bone formation and bone resorption and bone loss associated with estrogen deficiency occurs preferentially at sites experiencing low mechanical strain energies. The results of these studies suggest that estrogen and mechanical strain share common elements in their respective signal-transduction pathways. As a consequence, maintaining adequate exercise may reduce the effective dose of estrogen required for prevention of postmenopausal bone loss.
Acknowledgments
We thank Ms. Glenda Evans and Dr. Imke Schmidt for their technical assistance. We also thank Ms. Lori Rolbiecki for editorial and secretarial assistance. The spaceflight experiment was supported by Genetics Institute, Inc. (Cambridge, MA) and the Center for Cell Research, a National Aeronautics and Space Administration Center for the commercial development of space at the Pennsylvania State University (NAGW-1196). These studies were also supported by National Aeronautics and Space Administration Grant NAGW-4963, National Science Foundation Grant BIR937816, National Institutes of Health Grants AR41418, AR35651, and AG09241, and the Department of Veterans Affairs.
ABBREVIATIONS
- OVX
ovariectomy
- GAP
glyceraldehyde-3-phosphate dehydrogenase
- OC
osteocalcin
- USN
unilateral sciatic neurotomy
References
- 1.Richelson L S, Wahner H W, Melton L J, Riggs B L. N Engl J Med. 1984;311:1273–1275. doi: 10.1056/NEJM198411153112002. [DOI] [PubMed] [Google Scholar]
- 2.Aitken J M, Hart D M, Lindsay R. Br Med J. 1973;3:515–518. doi: 10.1136/bmj.3.5879.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsay R, Hart D M, Forrest C, Baird C. Lancet. 1980;ii:1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- 4.Compston J E, Yamaguchi K, Croucher P I, Garrahan N J, Lindsey P C, Shaw R W. Bone. 1995;16:261–267. doi: 10.1016/8756-3282(94)00038-2. [DOI] [PubMed] [Google Scholar]
- 5.Turner R T, Riggs B L, Spelsberg T C. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- 6.Wronski T J, Lowry P L, Walsh C C, Ignaszewski L A. Calcif Tissue Int. 1985;37:324–328. doi: 10.1007/BF02554882. [DOI] [PubMed] [Google Scholar]
- 7.Turner R T, Vandersteenhoven J J, Bell N H. J Bone Miner Res. 1987;2:115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 8.Kalu D N, Liu C C, Hardin R R, Hollis B W. Endocrinology. 1989;124:7–16. doi: 10.1210/endo-124-1-7. [DOI] [PubMed] [Google Scholar]
- 9.Goulding A, Gold E. J Endocrinol. 1989;121:293–298. doi: 10.1677/joe.0.1210293. [DOI] [PubMed] [Google Scholar]
- 10.Sibonga J D, Evans G L, Hauck E R, Bell N H, Turner R T. Breast Cancer Res Treat. 1996;41:71–79. doi: 10.1007/BF01807038. [DOI] [PubMed] [Google Scholar]
- 11.Powles T J, Hickish T F, Kanis J A, Ashley S. Proc Annu Mtg Am Soc Clin Oncol. 1995;14:A344. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 12.Steiniche T, Hasling C, Charles P, Eriksen E F, Mosekilde L, Melsen F. Bone. 1989;10:313–320. doi: 10.1016/8756-3282(89)90126-9. [DOI] [PubMed] [Google Scholar]
- 13.Heaney R P, Recker R R, Saville P D. J Lab Clin Med. 1978;92:964–970. [PubMed] [Google Scholar]
- 14.Wronski T J, Walsh C C, Ignaszewski L A. Bone. 1986;7:119–123. doi: 10.1016/8756-3282(86)90683-6. [DOI] [PubMed] [Google Scholar]
- 15.Riggs B L, Wahner H W, Dunn W C, Mazess R B, Offord K P, Melton L J. J Clin Invest. 1981;67:328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi H, Li M, Wronski T J. J Bone Miner Res. 1995;10:948–955. doi: 10.1002/jbmr.5650100616. [DOI] [PubMed] [Google Scholar]
- 17.Ke H Z, Jee W S S, Ito H, Setterberg R B, Li M, Lin B Y, Liang X, Ma Y F. Bone. 1993;14:481–485. doi: 10.1016/8756-3282(93)90183-b. [DOI] [PubMed] [Google Scholar]
- 18.Prince R L, Price R I, Ho S. J Bone Miner Res. 1988;3:305–310. doi: 10.1002/jbmr.5650030309. [DOI] [PubMed] [Google Scholar]
- 19.Leblanc A D, Schneider V S, Evans H J, Engelbretson D A, Krebs J M. J Bone Miner Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 20.Mack P B, LaChance P A, Vose G P, Vogt F B. Am J Roentgenol. 1967;100:503–511. doi: 10.2214/ajr.100.3.503. [DOI] [PubMed] [Google Scholar]
- 21.Elias A N, Gwinup G. J Am Paraplegia Soc. 1992;15:163–170. doi: 10.1080/01952307.1992.11735870. [DOI] [PubMed] [Google Scholar]
- 22.Page A, Jasty M, Bragdon C, Ito K, Harris W H. J Orthop Res. 1991;9:738–748. doi: 10.1002/jor.1100090514. [DOI] [PubMed] [Google Scholar]
- 23.Turner C H, Forwood M R, Rho J Y, Yoshikawa T. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 24.Chambers T J, Evans M, Gardner T N, Turner-Smith A, Chow J W. Bone Miner. 1993;20:167–178. doi: 10.1016/s0169-6009(08)80025-6. [DOI] [PubMed] [Google Scholar]
- 25.Zaman G, Dallas S L, Lanyon L E. Calcif Tissue Int. 1992;51:132–136. doi: 10.1007/BF00298501. [DOI] [PubMed] [Google Scholar]
- 26.Bagi C M, Mecham M, Weiss J, Miller S C. Bone. 1993;14:877–883. doi: 10.1016/8756-3282(93)90318-5. [DOI] [PubMed] [Google Scholar]
- 27.Westerlind K C, Wakley G K, Evans G L, Turner R T. Endocrinology. 1993;133:2924–2934. doi: 10.1210/endo.133.6.8243320. [DOI] [PubMed] [Google Scholar]
- 28.Westerlind K C, Wronski T J, Evans G L, Turner R T. Biochem Biophys Res Commun. 1994;200:283–289. doi: 10.1006/bbrc.1994.1446. [DOI] [PubMed] [Google Scholar]
- 29.Chomczynski P, Sacchi M. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 30.Celeste A J, Rosen V, Buecker J L, Kriz R, Wang E A, Wozney J M. Eur Mol Organ J. 1986;5:1885–1890. doi: 10.1002/j.1460-2075.1986.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genevese C, Rowe D, Kream B. Biochemistry. 1984;2:6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- 32.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferretti J L, Capozza R F, Mondelo N, Montuori E, Zanchetta J R. Bone. 1993;14:265–270. doi: 10.1016/8756-3282(93)90150-9. [DOI] [PubMed] [Google Scholar]
- 34.Weinans H, Huiskes R, Grootenboer H J. J Biomech. 1992;25:1425–1441. doi: 10.1016/0021-9290(92)90056-7. [DOI] [PubMed] [Google Scholar]
- 35.Flannery B P, Deckman H W, Roberge W G, D’Amico K L. Science. 1987;237:1439–1444. doi: 10.1126/science.237.4821.1439. [DOI] [PubMed] [Google Scholar]
- 36.Backup P, Westerlind K, Harris S, Spelsberg T, Kline B, Turner R T. Am J Physiol. 1994;266:E567–E573. doi: 10.1152/ajpendo.1994.266.4.E567. [DOI] [PubMed] [Google Scholar]
- 37.McDonald R, Hewgenaver J, Salman P. J Gerontol. 1986;41:445–452. doi: 10.1093/geronj/41.4.445. [DOI] [PubMed] [Google Scholar]
- 38.Lutz J, Chen F, Kasper C E. Aviat Space Environ Med. 1987;58:308–314. [PubMed] [Google Scholar]
- 39.Turner R T, Bell N H. J Bone Miner Res. 1986;1:399–407. doi: 10.1002/jbmr.5650010504. [DOI] [PubMed] [Google Scholar]
- 40.Wronski T J, Cintron M, Dann L M. Calcif Tissue Int. 1988;43:179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- 41.Frost H M. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 42.Fyhrie D P, Carter D R. J Orthop Res. 1986;4:304–317. doi: 10.1002/jor.1100040307. [DOI] [PubMed] [Google Scholar]
- 43.Carter D R, Orr J E, Fyhrie D P, Schurman D J. Clin Orthop. 1987;219:237–250. [PubMed] [Google Scholar]
- 44.Carter D R. J Biomech. 1987;20:1095–1109. doi: 10.1016/0021-9290(87)90027-3. [DOI] [PubMed] [Google Scholar]
- 45.Carter D R, Orr T E, Fyhrie D P. J Biomech. 1989;22:231–244. doi: 10.1016/0021-9290(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 46.Frost H M. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 47.Frost H M. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 48.Frost H M. Anat Rec. 1990;226:403–413. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]
- 49.Lanyon, L. E. (1992) J. Bone Miner. Res. 7, Suppl. 2, S369–S375. [DOI] [PubMed]
- 50.Rubin, C. T. (1984) Calcif. Tiss. Int. 36, Suppl. 1, S11–S18. [DOI] [PubMed]
- 51.Rubin C T, Lanyon L E. J Exp Biol. 1982;101:187–211. doi: 10.1242/jeb.101.1.187. [DOI] [PubMed] [Google Scholar]
- 52.Rodan G A. J Bone Miner Res. 1989;6:527–530. doi: 10.1002/jbmr.5650060602. [DOI] [PubMed] [Google Scholar]
- 53.Dempster D W, Birchman R, Xu R, Lindsay R, Shen V. Bone. 1995;16:157–161. [PubMed] [Google Scholar]
- 54.Turner R T. J Bone Miner Res. 1994;9:1419–1424. doi: 10.1002/jbmr.5650090913. [DOI] [PubMed] [Google Scholar]
- 55.Tuukkanen J, Peng Z, Väänänen H K. Calcif Tissue Int. 1991;49:S80. doi: 10.1007/BF02555098. [DOI] [PubMed] [Google Scholar]
- 56.Barengolts E I, Curry D J, Bapna M S, Kukreja S C. Calcif Tissue Int. 1993;52:239–243. doi: 10.1007/BF00298726. [DOI] [PubMed] [Google Scholar]
- 57.Ellinger G M, Duckworth J, Dalgarno A C, Quenouille M H. Br J Nutr. 1952;6:235–253. doi: 10.1079/bjn19520027. [DOI] [PubMed] [Google Scholar]
- 58.Brommage R, DeLuca H F. Am J Physiol. 1985;248:E182–E187. doi: 10.1152/ajpendo.1985.248.2.E182. [DOI] [PubMed] [Google Scholar]