Abstract
beta-Lactamase with the -20 to -1 region of the leader peptide deleted (almost complete deletion of the leader peptide) [delta(-20,-1) beta-lactamase] was released from Escherichia coli cells by osmotic shock. Fractionation of the cells by conversion to spheroplasts and protease accessibility experiments further indicated that a portion of the protein may be bound to the cytoplasmic membrane and be partially exposed in the periplasmic space. Expression of delta(-20,-1) beta-lactamase conferred a 25-fold increase in the 50% lethal dose for ampicillin relative to that for controls, thus confirming that a small amount (about 2%) of the active protein is completely exported from the cytoplasm. These results suggest that even in the absence of a leader peptide, mature beta-lactamase is able to interact with the cytoplasmic membrane and be translocated into the periplasmic space, albeit with a low efficiency.
Full text
PDF
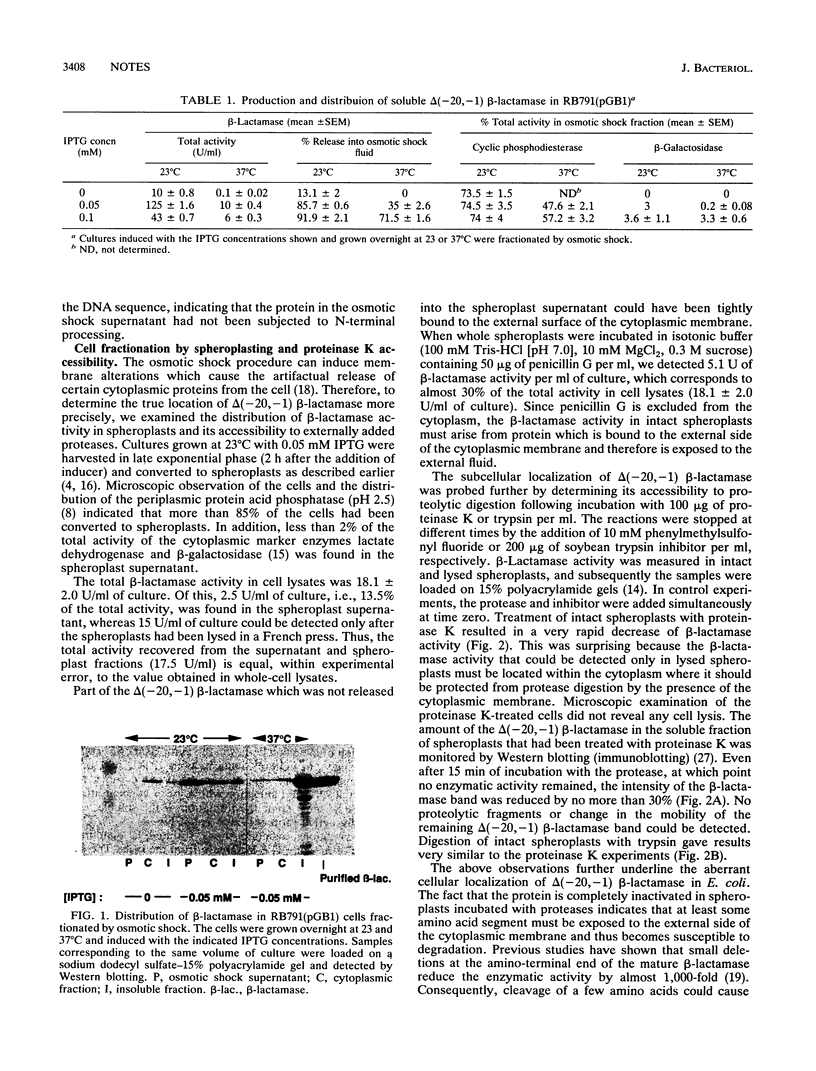


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baneyx F., Georgiou G. Construction and characterization of Escherichia coli strains deficient in multiple secreted proteases: protease III degrades high-molecular-weight substrates in vivo. J Bacteriol. 1991 Apr;173(8):2696–2703. doi: 10.1128/jb.173.8.2696-2703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H., Lunn C. A., Pigiet V. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. A., Georgiou G. Folding and aggregation of beta-lactamase in the periplasmic space of Escherichia coli. J Biol Chem. 1990 Oct 5;265(28):16760–16766. [PubMed] [Google Scholar]
- Bowden G. A., Paredes A. M., Georgiou G. Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology (N Y) 1991 Aug;9(8):725–730. doi: 10.1038/nbt0891-725. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. A vector for the construction of translational fusions to TEM beta-lactamase and the analysis of protein export signals and membrane protein topology. Gene. 1986;49(3):341–349. doi: 10.1016/0378-1119(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Chalmers J. J., Kim E., Telford J. N., Wong E. Y., Tacon W. C., Shuler M. L., Wilson D. B. Effects of temperature on Escherichia coli overproducing beta-lactamase or human epidermal growth factor. Appl Environ Microbiol. 1990 Jan;56(1):104–111. doi: 10.1128/aem.56.1.104-111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E., Boquet P. L. ExpA: a conditional mutation affecting the expression of a group of exported proteins in Escherichia coli K-12. Mol Gen Genet. 1981;181(2):192–200. doi: 10.1007/BF00268426. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Brockman R. W., Heppel L. A. Purification and properties of two acid phosphatase fractions isolated from osmotic shock fluid of Escherichia coli. Biochemistry. 1967 Jun;6(6):1743–1751. doi: 10.1021/bi00858a024. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Randall L. L. A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science. 1991 Jan 25;251(4992):439–443. doi: 10.1126/science.1989077. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Takacs B. J., Rosenbusch J. P. Properties of a major protein released from Escherichia coli by osmotic shock. Biochemistry. 1976 Jun 1;15(11):2297–2303. doi: 10.1021/bi00656a008. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Plückthun A., Knowles J. R. Signal sequence mutants of beta-lactamase. J Biol Chem. 1985 Dec 25;260(30):16192–16199. [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. Evidence for partial export of Vitreoscilla hemoglobin into the periplasmic space in Escherichia coli. Implications for protein function. J Mol Biol. 1989 Nov 5;210(1):79–89. doi: 10.1016/0022-2836(89)90292-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minsky A., Summers R. G., Knowles J. R. Secretion of beta-lactamase into the periplasm of Escherichia coli: evidence for a distinct release step associated with a conformational change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4180–4184. doi: 10.1073/pnas.83.12.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pugsley A. P. On remaining cytoplasmic. Biochimie. 1990 Feb-Mar;72(2-3):89–94. doi: 10.1016/0300-9084(90)90133-2. [DOI] [PubMed] [Google Scholar]
- Puziss J. W., Strobel S. M., Bassford P. J., Jr Export of maltose-binding protein species with altered charge distribution surrounding the signal peptide hydrophobic core in Escherichia coli cells harboring prl suppressor mutations. J Bacteriol. 1992 Jan;174(1):92–101. doi: 10.1128/jb.174.1.92-101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Rosenwasser T. A., Hogquist K. A., Nothwehr S. F., Bradford-Goldberg S., Olins P. O., Chaplin D. D., Gordon J. I. Compartmentalization of mammalian proteins produced in Escherichia coli. J Biol Chem. 1990 Aug 5;265(22):13066–13073. [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Broome-Smith J. K. Identification of amino acid sequences that can function as translocators of beta-lactamase in Escherichia coli. Mol Microbiol. 1989 Oct;3(10):1361–1369. doi: 10.1111/j.1365-2958.1989.tb00117.x. [DOI] [PubMed] [Google Scholar]





