Abstract
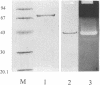
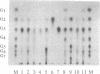
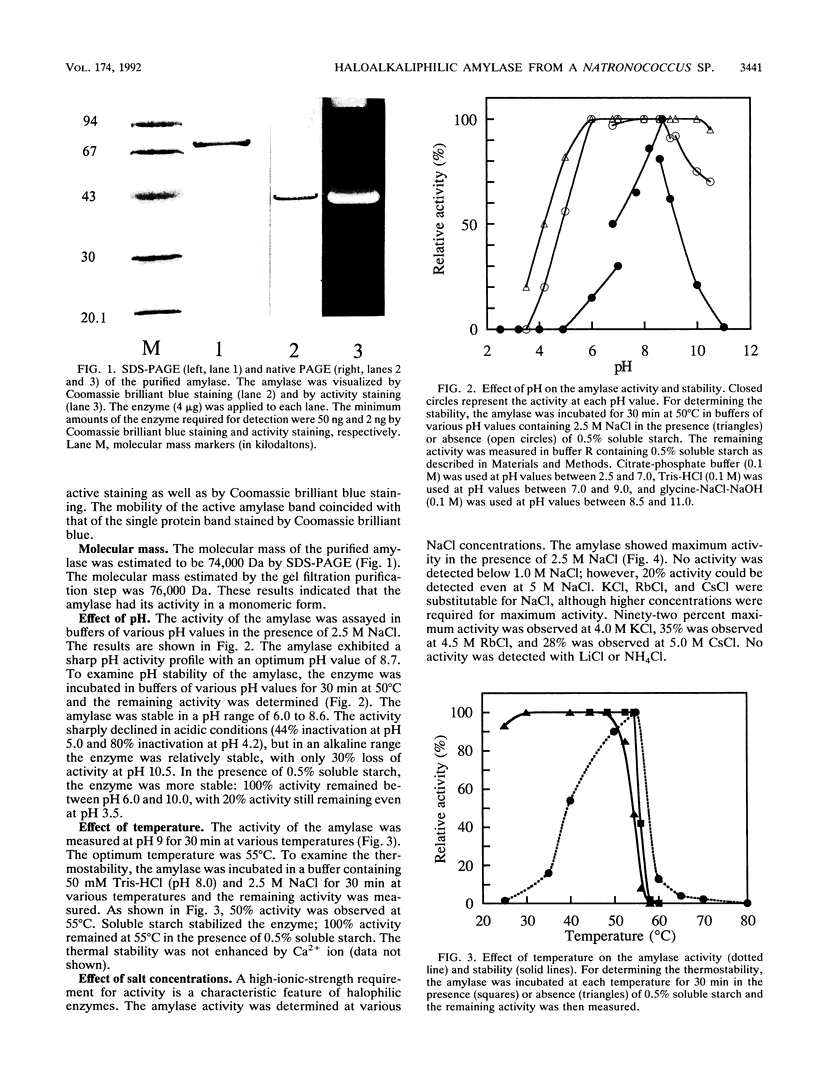
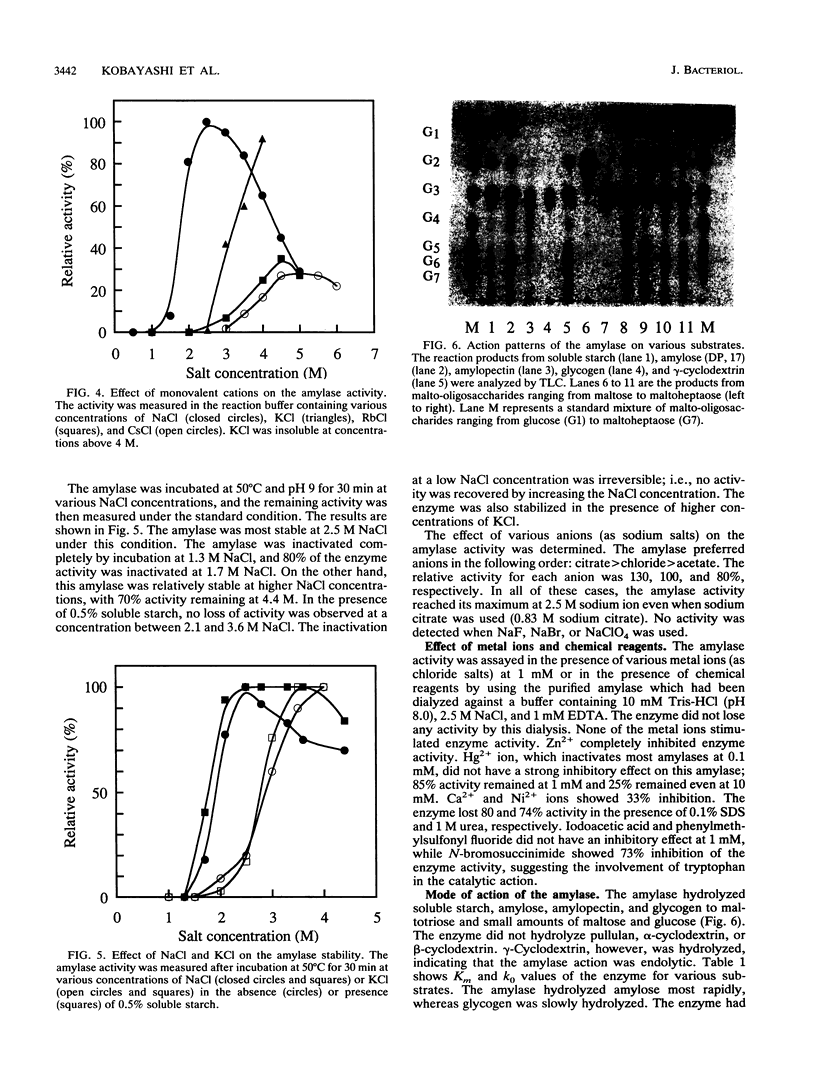
A haloalkaliphilic archaebacterium, Natronococcus sp. strain Ah-36, produced extracellularly a maltotriose-forming amylase. The amylase was purified to homogeneity by ethanol precipitation, hydroxylapatite chromatography, hydrophobic chromatography, and gel filtration. The molecular weight of the enzyme was estimated to be 74,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The amylase exhibited maximal activity at pH 8.7 and 55 degrees C in the presence of 2.5 M NaCl. The activity was irreversibly lost at low ionic strength. KCl, RbCl, and CsCl could partially substitute for NaCl at higher concentrations. The amylase was stable in the range of pH 6.0 to 8.6 and up to 50 degrees C in the presence of 2.5 M NaCl. Stabilization of the enzyme by soluble starch was observed in all cases. The enzyme activity was inhibited by the addition of 1 mM ZnCl2 or 1 mM N-bromosuccinimide. The amylase hydrolyzed soluble starch, amylose, amylopectin, and, more slowly, glycogen to produce maltotriose with small amounts of maltose and glucose of an alpha-configuration. Malto-oligosaccharides ranging from maltotetraose to maltoheptaose were also hydrolyzed; however, maltotriose and maltose were not hydrolyzed even with a prolonged reaction time. Transferase activity was detected by using maltotetraose or maltopentaose as a substrate. The amylase hydrolyzed gamma-cyclodextrin. alpha-Cyclodextrin and beta-cyclodextrin, however, were not hydrolyzed, although these compounds acted as competitive inhibitors to the amylase activity. Amino acid analysis showed that the amylase was characteristically enriched in glutamic acid or glutamine and in glycine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Good W. A., Hartman P. A. Properties of the amylase from Halobacterium halobium. J Bacteriol. 1970 Oct;104(1):601–603. doi: 10.1128/jb.104.1.601-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izotova L. S., Strongin A. Y., Chekulaeva L. N., Sterkin V. E., Ostoslavskaya V. I., Lyublinskaya L. A., Timokhina E. A., Stepanov V. M. Purification and properties of serine protease from Halobacterium halobium. J Bacteriol. 1983 Aug;155(2):826–830. doi: 10.1128/jb.155.2.826-830.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainuma K., Wako K., Kobayashi S., Nogami A., Suzuki S. Purification and some properties of a novel maltohexaose-producing exo-amylase from Aerobacter aerogenes. Biochim Biophys Acta. 1975 Dec 18;410(2):333–346. doi: 10.1016/0005-2744(75)90235-1. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. Halophilic amylase from a moderately halophilic Micrococcus. J Bacteriol. 1972 Feb;109(2):570–574. doi: 10.1128/jb.109.2.570-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. PURIFICATION AND ACTION PATTERN OF AN AMYLASE FROM BACILLUS POLYMYXA. Arch Biochem Biophys. 1964 Feb;104:338–345. doi: 10.1016/s0003-9861(64)80024-2. [DOI] [PubMed] [Google Scholar]
- Rao J. K., Argos P. Structural stability of halophilic proteins. Biochemistry. 1981 Nov 10;20(23):6536–6543. doi: 10.1021/bi00526a004. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J. Isolation, purification, and characterization of a maltotetraose-producing amylase from Pseudomonas stutzeri. Arch Biochem Biophys. 1971 Jul;145(1):105–114. doi: 10.1016/0003-9861(71)90015-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Ohmura K., Nakayama A., Takeichi Y., Otozai K., Yamasaki M., Tamura G., Yamane K. Alpha-amylase genes (amyR2 and amyE+) from an alpha-amylase-hyperproducing Bacillus subtilis strain: molecular cloning and nucleotide sequences. J Bacteriol. 1983 Oct;156(1):327–337. doi: 10.1128/jb.156.1.327-337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]