Abstract
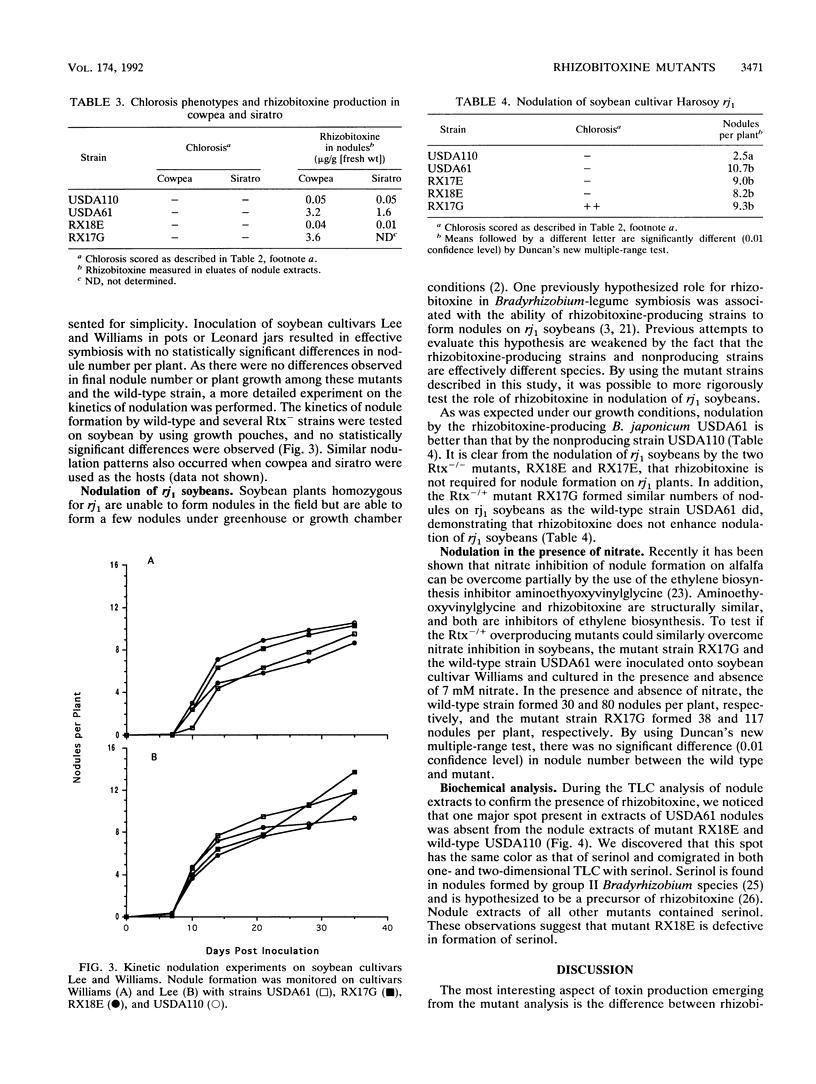
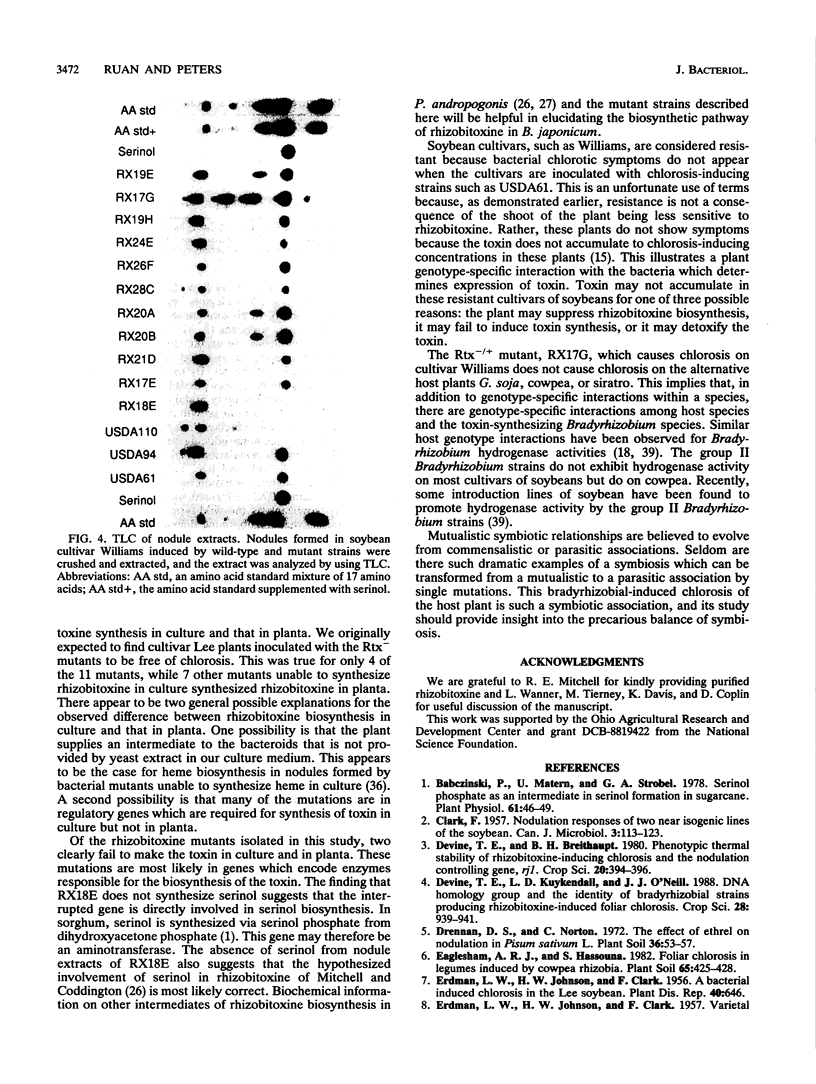
To explore the role of rhizobitoxine in Bradyrhizobium-legume symbiosis, 11 rhizobitoxine mutants of B. japonicum USDA61 were isolated on the basis of their inability to synthesize the toxin in culture. Each mutant is prototrophic and symbiotically effective on soybean, cowpea, siratro, and Glycine soja. The rhizobitoxine mutants differ in their chlorosis phenotypes and rhizobitoxine production in planta. As expected, one group of mutant fail to make toxin in planta, resulting in the absence of chlorosis. Another group of mutants causes severe chlorosis on all cultivars of soybean tested. Surprisingly, this group of mutants makes more rhizobitoxine in soybean nodules than the wild-type strain does. This phenotype is only observed on soybean and not on other hosts such as cowpea, siratro, or G. soja. The remaining mutants all produce rhizobitoxine in planta but vary in the amount of toxin they produce and the severity of chlorosis they induce in soybean plants. Biochemical analysis of mutants demonstrates that one mutant is unable to synthesize serinol, a molecule hypothesized to be an intermediate in rhizobitoxine biosynthesis. By using these mutants, it was found that rhizobitoxine plays no apparent role in the nodulation of rj1 soybeans. Recently, it was found that inhibition of ethylene biosynthesis allows Rhizobium meliloti to overcome nitrate inhibition of nodule formation on alfalfa. Because rhizobitoxine also inhibits ethylene biosynthesis, we tested the ability of mutants which accumulate high levels of toxin in planta to overcome nitrate inhibition of nodule formation on soybean plants and found that the nodule formation induced by the wild type and that induced by mutant strains were equally suppressed in the presence of nitrate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babczinski P., Matern U., Strobel G. A. Serinol phosphate as an intermediate in serinol formation in sugarcane. Plant Physiol. 1978 Jan;61(1):46–49. doi: 10.1104/pp.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearn J. C., Larue T. A. Ethylene Inhibitors Restore Nodulation to sym 5 Mutants of Pisum sativum L. cv Sparkle. Plant Physiol. 1991 May;96(1):239–244. doi: 10.1104/pp.96.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. Mechanism of inhibition of spinach beta-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971 Mar 10;227(3):671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., van Berkum P., Weber D. F. A Comparative Study of the Physiology of Symbioses Formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol. 1982 Dec;70(6):1626–1630. doi: 10.1104/pp.70.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Ligero F., Caba J. M., Lluch C., Olivares J. Nitrate inhibition of nodulation can be overcome by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1991 Nov;97(3):1221–1225. doi: 10.1104/pp.97.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Guggenheim S., Hilton J. L. Rhizobium-synthesized phytototoxin: an inhibitor of beta-cystathionase in Salmonella typhimurium. Biochim Biophys Acta. 1968 May;158(2):219–225. doi: 10.1016/0304-4165(68)90134-7. [DOI] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Production of the Soybean-Chlorosis Toxin by Rhizobium japonicum in Pure Culture. Plant Physiol. 1965 Sep;40(5):931–933. doi: 10.1104/pp.40.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Rhizobial-Induced Chlorosis in Soybeans: Isolation, Production in Nodules, and Varietal Specificity of the Toxin. Plant Physiol. 1965 Sep;40(5):927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Crist-Estes D. K. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1989 Oct;91(2):690–693. doi: 10.1104/pp.91.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Peters N. K. Rapid and sensitive assay for the phytotoxin rhizobitoxine. Appl Environ Microbiol. 1991 Jul;57(7):2097–2100. doi: 10.1128/aem.57.7.2097-2100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I., O'brian M. R. Evidence for an inter-organismic heme biosynthetic pathway in symbiotic soybean root nodules. Science. 1991 Mar 8;251(4998):1220–1222. doi: 10.1126/science.251.4998.1220. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- van Berkum Peter, Sloger Charles. Hydrogen Oxidation by the Host-Controlled Uptake Hydrogenase Phenotype of Bradyrhizobium japonicum in Symbiosis with Soybean Host Plants. Appl Environ Microbiol. 1991 Jun;57(6):1863–1865. doi: 10.1128/aem.57.6.1863-1865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]