Abstract
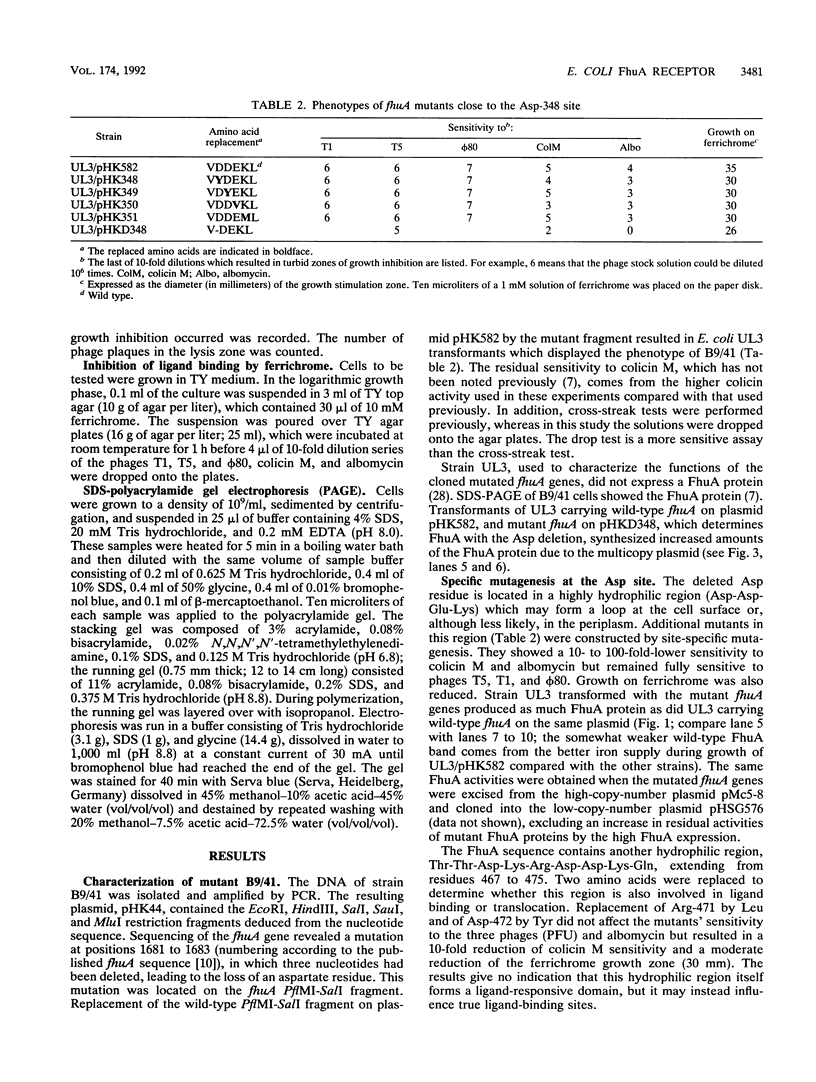
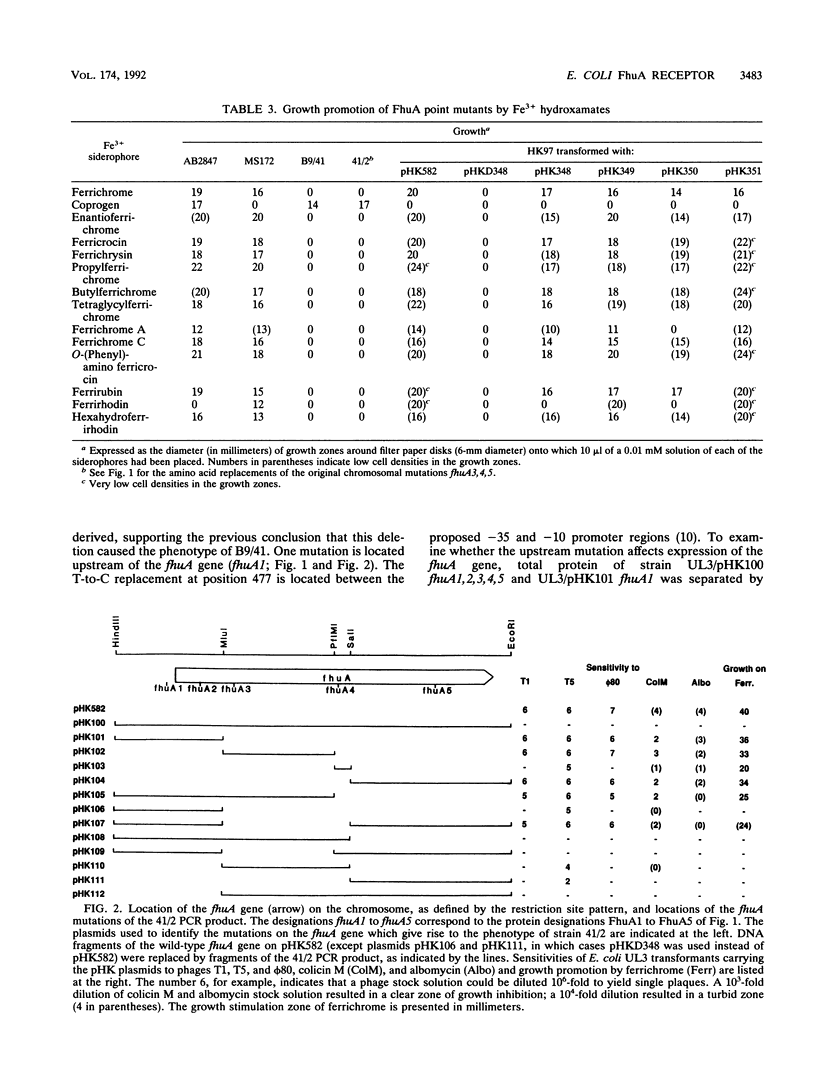
The FhuA protein of the outer membrane serves as a receptor for phages T5, T1, and phi 80, for colicin M, for the antibiotic albomycin, and for ferrichrome and related siderophores. To identify protein regions important for the multiple FhuA activities, fhuA genes of spontaneous chromosomal mutants which expressed wild-type amounts of the FhuA protein were sequenced. A mutant which was partially T5 sensitive but impaired in all other functions was missing aspartate residue 348 of the mature protein as a result of a three-base deletion. This aspartate residue is part of the hydrophilic sequence Asp-Asp-Glu-Lys. Replacement by site-specific mutagenesis of each of the Asp residues by Tyr, of Glu by Val, and of Lys by Met reduced FhuA activity but less than the Asp deletion did. Ferrichrome inhibited binding of phage phi 80 and of colicin M to these mutants in an allele-specific manner. A completely resistant derivative of the Asp deletion mutant contained, in addition, a leucine-to-proline substitution at position 106 and eight changed bases, converting at positions 576 to 578 an Arg-Pro-Leu sequence to Ala-Arg-Cys. The latter mutations and the Leu-to-Pro replacement alone did not alter sensitivity to the phages but reduced sensitivity to colicin M and albomycin 10- to 1,000-fold. The proline replacements probably disturb FhuA conformation and, in concert with the Asp deletion, inactivate FhuA completely. It is concluded that the Asp deletion site defines a region of FhuA which directly participates in binding of all FhuA ligands. Growth promotion studies on iron-limited media revealed that certain siderophores of the hydroxamate type, such as butylferrichrome, ferrichrysin, and ferrirubin, are taken up not only via FhuA but also via the FhuE outer membrane receptor protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. K., Francis C. L., McIntosh M. A. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J Biol Chem. 1990 Aug 25;265(24):14536–14543. [PubMed] [Google Scholar]
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Braun V. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J Bacteriol. 1989 Nov;171(11):6387–6390. doi: 10.1128/jb.171.11.6387-6390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Coulton J. W. Internal deletions in the FhuA receptor of Escherichia coli K-12 define domains of ligand interactions. J Bacteriol. 1991 Jul;173(14):4394–4403. doi: 10.1128/jb.173.14.4394-4403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Hellstern D., Henning D., Coulton J. W. Insertion mutagenesis of the gene encoding the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1990 Apr;172(4):1861–1869. doi: 10.1128/jb.172.4.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bradbeer C., Kadner R. J. Altered binding and transport of vitamin B12 resulting from insertion mutations in the Escherichia coli btuB gene. J Biol Chem. 1988 Oct 5;263(28):14224–14230. [PubMed] [Google Scholar]
- Günter K., Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990 Nov 12;274(1-2):85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- Günter K., Braun V. Probing FhuA'-'PhoA fusion proteins for the study of FhuA export into the cell envelope of Escherichia coli K12. Mol Gen Genet. 1988 Dec;215(1):69–75. doi: 10.1007/BF00331305. [DOI] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K12. Mol Gen Genet. 1983;191(2):301–306. doi: 10.1007/BF00334830. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Holland I. B. Intermediates in the assembly of the TonA polypeptide into the outer membrane of Escherichia coli K12. J Mol Biol. 1986 Jun 5;189(3):477–486. doi: 10.1016/0022-2836(86)90318-9. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990 Dec;4(12):2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol. 1990 Mar;4(3):427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Schultz G., Ullrich F., Heller K. J., Braun V. Export and activity of hybrid FhuA'-'Iut receptor proteins and of truncated FhuA' proteins of the outer membrane of Escherichia coli. Mol Gen Genet. 1989 Apr;216(2-3):230–238. doi: 10.1007/BF00334361. [DOI] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: application to the study of periplasmic permeases. J Bacteriol. 1989 Mar;171(3):1602–1608. doi: 10.1128/jb.171.3.1602-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Winkelmann G. Structural and stereochemical aspects of iron transport in fungi. Biotechnol Adv. 1990;8(1):207–231. doi: 10.1016/0734-9750(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Zhou C., Yang Y., Jong A. Y. Mini-prep in ten minutes. Biotechniques. 1990 Feb;8(2):172–173. [PubMed] [Google Scholar]