Abstract
A primary muscle cell culture derived from newborn rabbit muscle and growing on microcarriers in suspension was established. When cultured for several weeks, the myotubes in this model develop the completely adult pattern of fast myosin light and heavy chains. When Ca2+ ionophore is added to the culture medium on day 11, raising intracellular [Ca2+] about 10-fold, the myotubes develop to exhibit properties of an adult slow muscle by day 30, expressing slow myosin light as well as heavy chains, elevated citrate synthase, and reduced lactate dehydrogenase. The remarkable plasticity of these myotubes becomes apparent, when 8 days after withdrawal of the ionophore a marked slow-to-fast transition, as judged from the expression of isomyosins and metabolic enzymes, occurs.
While the alterations that occur in mammalian muscle during fast-to-slow transition in vivo are known in great detail, mostly from chronic electrostimulation experiments with fast hindlimb muscles (1), the information on the mechanism initiating this process is sparse. A reduced intracellular phosphorylation potential and an elevated intracellular Ca2+ concentration ([Ca2+]i), as they occur during sustained contractile activity, have been discussed as possible trigger events (2–5). It was our aim to test whether an increase in [Ca2+]i imposed on the muscle cell is indeed able to induce a fast-to-slow transition. Because such an experiment cannot be performed in vivo, we were searching for a suitable myogenic cell culture system in which this would become feasible.
Thus, this paper has two goals: (i) to report how a primary muscle culture can be grown that develops into an adult-like state, expressing adult myosin of the fast type only, and (ii) to study whether manipulation of [Ca2+]i induces a shift between “fast” and “slow” fiber properties in the cultured myotubes. We show, first, that myotubes derived from newborn rabbit muscles and grown for 4 weeks on microcarriers in suspension possess a purely adult fast myosin pattern. Second, we show that these myotubes exhibit a similarly remarkable plasticity as it is known for adult rabbit muscles (1). This plasticity becomes apparent during exposure of the myotubes to high [Ca2+]i, which causes development of the slow isoforms of myosin light chains (MLCs) and myosin heavy chains (MHCs) instead of their fast counterparts, of an increased aerobic metabolic capacity accompanied by a decreased anaerobic capacity as evidenced from elevated citrate synthase (CS) and reduced lactate dehydrogenase (LDH) levels, and of an increased activity ratio of the carbonic anhydrases CA III/CA II. Lowering [Ca2+]i back to normal levels is followed by a reversal of all these changes within a few days.
EXPERIMENTAL PROCEDURES
Culture and Harvesting of Muscle Cells.
Newborn White New Zealand rabbits were killed by decapitation. Hindlimb muscles were cut in small pieces and incubated in BSS, pH 7.0 (4.56 mM KCl/0.44 mM KH2PO4/0.42 mM Na2HPO4/25 mM NaHCO3/119.8 mM NaCl/50 mg/liter penicillin/100 mg/liter streptomycin) with 0.125% trypsin under stirring at 37°C for 1 h. The suspension was centrifuged at 800 × g for 5 min, the pellet resuspended in DMEM with 10% neonatal calf serum (NCS), and the entire procedure repeated once. The final pellet was suspended in DMEM/10% NCS and then filtered through a sieve with 0.4-mm pores. The filtrate was transferred into culture bottles where the fibroblasts were allowed to settle and attach themselves to the bottom for 30 min. The supernatant suspension was decanted and diluted to a final cell density of 8 × 105/ml in DMEM with 10% NCS. A total of 15 ml of this suspension were filled into one 260-ml culture flask and 0.04 g cross-linked gelatin beads with a diameter of 100–300 μm (CultiSpher-GL; Percell Biolytica, Astorp, Sweden) were added per flask. The flasks were kept at 37°C in 8% CO2 in air and 95% humidity while being shaken gently to ensure adequate O2 supply to the cells and to prevent cells and beads from settling down. Twenty-four hours later the cell suspension was diluted to a cell concentration of 4 × 105 cells/ml. Myoblasts attached themselves to the gelatin beads and began to fuse after 3 days in culture. After 2 weeks fusion appeared to be complete and only myotubes were detectable. To collect the myotubes after 3–5 weeks of culture, cell-covered beads were allowed to sediment, washed twice in BSS (pH 7.0) with 0.02% EDTA, and resuspended in BSS (pH 7.9) containing 0.35% trypsin, 1.8 mM CaCl2, and 0.8 mM MgSO4. After incubation for 30 min at 37°C under shaking, the isolated cells were spun down at 800 × g for 5 min, washed twice in BSS (pH 7.0) with 0.02% EDTA, and then suspended in BSS (pH 7.0). This suspension was sonicated 6 × 5 s with 60 W at 0°C.
Scanning Electron Microscopy.
The carrier suspension is pipetted onto a collagen-coated glass slide and incubated for 3 hr in a chamber saturated with water vapor. Thereafter the carriers are firmly attached to the slide and are washed two times with 0.1 M cacodylate (pH 7.3). Glutardialdehyde (2.5%) is pipetted onto the carriers, and the slides are incubated again for 2 hr in a humid chamber for fixation, then again rinsed in cacodylate buffer. This is followed by standard treatment for scanning electron microscopy (6).
MLC Electrophoresis.
Cell culture homogenates were centrifuged at 100,000 × g for 1 hr and the pellets were incubated in extraction buffer with 0.6 M KCl, 10 mM EGTA, 0.5 mM dithiotreitol, 1 mM phenylmethylsulfonyl fluoride, 10 mM phosphate (pH 6.8) (1:7 vol/vol) for 1 hr at 4°C. After centrifugation at 10,000 × g for 10 min, the supernatant was diluted 1:10 with ice-cold water to precipitate actomyosin over night at 0°C. After centrifugation at 20,000 × g the pellet was solubilized with extraction buffer, mixed 1:1 with glycerol, and stored at −20°C until used. MLC isoforms were analyzed by two-dimensional electrophoresis as described by O’Farrell (7). The first dimension was carried out by isoelectric focusing of 10 μg protein aliquots of myosin extract solubilized in O’Farrell’s lysis buffer. The 5% slab gel contained 9 M urea and 2% ampholyte (pH 3.5–9.5). After focusing, stripes were cut out and equilibrated in 50 mM Tris·HCl (pH 6.8), 9 M urea, 30% glycerol, 3% SDS, and 0.25% dithiotreitol. The second dimension was then carried out in a 5% stacking and 15% separating gel. Thereafter gels were silver-stained.
MHC Electrophoresis.
MHC electrophoresis was carried out on myosin extracts using a modification of the method of Carraro and Catani (8) as described (17) using a slab gel with a 3.5% stacking (pH 6.8) and a 6.6% separating gel (pH 8.8) followed by a second 6.6% stacking (pH 6.8) with a second 8.8% separating gel (pH 8.8). The latter three gels contained 4.8%, 14.5%, and 24.5% glycerol, respectively. After runs, gels were silver stained according to Heukeshoven and Dernick (9).
[Ca2+]i Measurements.
[Ca2+]i concentrations were determined by loading myotubes with Fura-2 AM. The optical measurement was performed in a microscope photometer over single myotubes as described (10). By successive excitation of the dye with light at wavelengths of 340 and 380 nm and measuring the emitted light at 510 ± 10 nm, the ratio, R340/380, of light intensities emitted at the two excitation wavelengths was obtained. This ratio is related to the [Ca2+]i in the myotubes.
CA Activities.
CA activities were measured using the barbital system in Maren’s micromethod as described by Bruns and Gros (11). CA III activity was determined in the presence of 5 × 10−6 M of the CA inhibitor acetazolamide, and CA II activity was obtained by subtracting CA III activity from the total activity measured in the absence of inhibitor (12).
CS, LDH, and Creatine Kinase (CK).
These enzymes were determined using standard optically coupled tests (13, 14).
RESULTS AND DISCUSSION
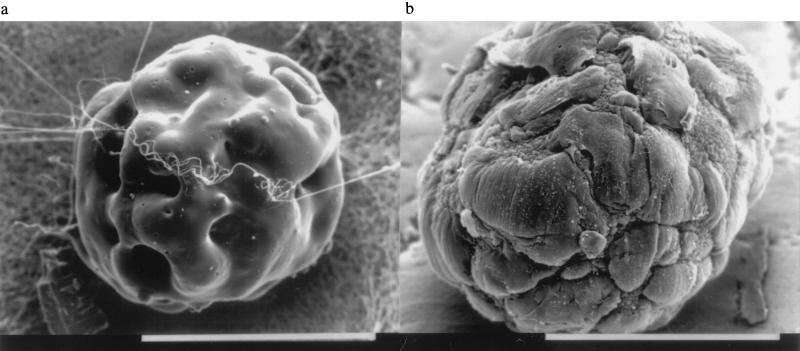
Shahar and Reuveny (15) demonstrated that skeletal muscle cultures can be grown on microcarriers. The present newborn rabbit-derived myoblasts show a strong tendency to fuse to give myotubes under normal culture conditions in the gelatin bead culture used here. Fig. 1a shows a scanning electron micrograph of the type of gelatin beads employed. Their surface is quite irregular and characterized by deep valleys and holes. Fig. 1b shows a myotube-covered gelatin bead after 28 days in culture. Practically all cells on the beads are myotubes at this age with very few myoblasts. Many myotubes extend across the “mountains” of the bead surface and their ends are fixed to the valleys and holes. These myotube cultures could be held for up to 5 weeks in a vital and increasingly mature state as judged from the expression of muscle-specific proteins and morphological properties such as the appearance of T-tubule openings on the sarcolemmal surface (the latter are not visible at the magnification of Fig. 1b). Preliminary experiments with beads possessing smoother surfaces had shown an early detachment of the myotubes from the beads. It may be noted that the myotubes contracted vigorously and spontaneously during the first two weeks, whereupon spontaneous contraction began to disappear. This is perhaps, in conjunction with the appearance of a pure adult myosin pattern and the development of T-tubules, another sign of increased maturity of the myotubes in the later stages of this culture.
Figure 1.
Scanning electron micrograph of (a) the gelatin bead employed in the culture system (CultiSpher-GL), and (b) a bead covered with myotubes on day 28 of the culture. (Bars = 50 μm.)
We have also grown primary muscle cultures from the same source and using the same medium in collagen-coated Petri dishes. Here, long cylindrical myotubes develop in a manner similar, for example, to figure 17 of Nag and Foster (16). When the myotubes begin to contract, their cell body, except the very ends of the multinucleated cell, detaches from the collagen layer of the dish. Five days after they have begun to contract they are lost, probably because the increasing contractile force disrupts the attachment of the myotube endings to the collagen. They are then replaced by newly formed myotubes. Thus, in contrast to the bead culture, in which a virtually complete fusion and a myotube age of several weeks are achieved, the conventional Petri dish culture is characterized by a constant disappearance and reformation of myotubes with the lasting presence of a major number of myoblasts and a myotube age that does not exceed a few days.
Time Course of Isomyosin Expression in Culture.
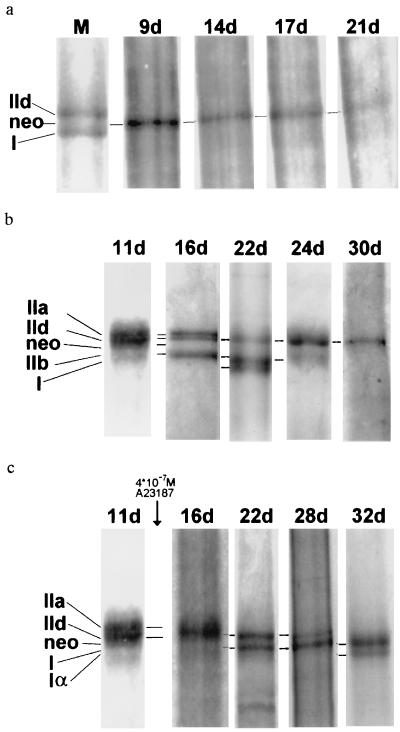
We have previously shown that with the present electrophoretic technique MHC isoforms can be separated with good resolution and appear on the SDS gel in the sequence MHCemb < MHC IIa < MHC IId < MHCneo < MHC IIb < MHC I < MHC Iα (17). Here, MHCemb is the embryonic isoform; MHCneo the neonatal form; MHC IIa, IIb, and IId are fast adult isoforms; MHC I is the slow isoform of adult skeletal muscle; and MHC Iα is one of the slow cardiac forms. Fig. 2 shows MHC expression versus time under different culture conditions. In Fig. 2a conventional Petri dishes were used to grow primary rabbit muscle cultures. Fig. 2a shows SDS electrophoreses of MHC from these cultures at different ages. They demonstrate that after 9 days in culture essentially only the neonatal MHC, MHCneo, is expressed, and this situation remains unchanged until day 21. The myotubes remain “locked” in a neonatal state, presumably because they do not become old enough to grow into a more mature state. This is in accordance with the MLC pattern of these cultures that we find in the two-dimensional electrophoresis (not shown), which exhibits a combination of the adult fast MLC isoforms with an increasing fraction of the embryonic isoform LCemb, an observation that agrees well with the literature (18, 19). In conclusion, the muscle primary culture grown in Petri dishes remains in an immature state when judged by its MHC as well as by its MLC pattern.
Figure 2.
Time course of MHC pattern development in primary rabbit muscle cultures. SDS/PAGE of myosin extracts. M, marker lane; times given are age of cultures in days. (a) Culture growing in conventional collagen-coated Petri dishes. Marker lane: mixture of myosin extracts from rabbit soleus and tibialis anterior. (b) Culture growing on gelatin beads. (c) Culture growing on gelatin beads. After day 11 Ca2+ ionophore A23187 was present in the culture medium at a concentration of 4 × 10−7M. It may be noted that b represents an improvement over c in terms of separating distances between the bands due to a longer running time of the gel at lower temperature. Note also that gels in b and c were aligned using the position of markers such as that seen in a. A marker lane was present in every gel run.
A very different development of the myosin pattern is seen when the rabbit muscle primary culture is grown on beads in suspension. Fig. 2b shows a time course of the MHC pattern as it is apparent in SDS/PAGE. On day 11 the myotubes exhibit a complex, and poorly resolved, mixture of adult and neonatal MHC isoforms. On day 16 the nonadult isoform has disappeared and we have MHC IIa, MHC IId, and MHC IIb. After a transitory shift toward the slow isoforms on day 22, on day 24 almost only and on day 30 only the adult fast MHC IId is observed, which is the dominating MHC of many fast adult rabbit muscles (e.g., tibialis anterior, extensor digitorum longus, plantaris, psoas). Other examples with a similar sequence of patterns are shown in Figs. 5 and 6 Lower (control). To our knowledge, this is the first description of a myogenic culture which develops to express exclusively adult MHC isoforms. Although previously disputed by several authors, it has been shown by some (20–23) that, besides embryonic and neonatal forms, to some extent adult MHC or adult MHC mRNA can be expressed by myogenic cell lines, satellite or neonatal myoblast cultures under simple culture conditions without coculture with nerve or connective tissue. In fact, Weydert et al. (21) have shown that in three myogenic cell lines initially very small fractions of MHC transcripts represent the adult forms, but these fractions increase with culture time and they are constituted by the fast MHC isoform. This would lend further support to the idea that, whether the adult state is reached in myotubes, depends to a large extent on the age reached by these myotubes.
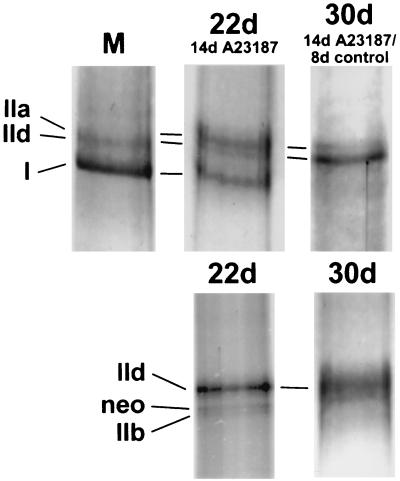
Figure 5.
Reversibility of the effect of ionophore treatment on MHC pattern of myotubes by SDS/PAGE. (Upper) Day 22, MHCs after ionophore exposure for 14 days; day 30, MHCs after ionophore withdrawal for 8 days. (Lower) Controls without ionophore. Marker lane (M): mixture of myosin extracts from soleus and tibialis anterior of the rabbit.
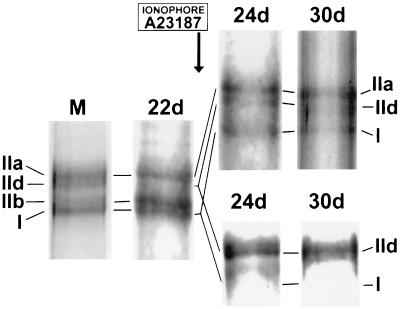
Figure 6.
Effects of late addition of Ca2+ ionophore to the cultures on MHC patterns by SDS/PAGE. (Upper) MHC patterns after addition of the ionophore from day 22 on. (Lower) Controls without ionophore. Marker lane (M): mixture of myosin extracts from tongue muscle, soleus, and young masseter of the rabbit.
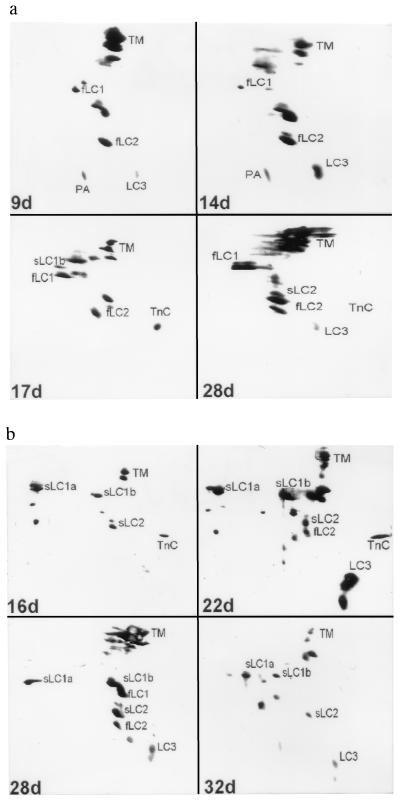
In line with this observation, the MLC pattern of the bead culture (Fig. 3a) shows at all stages the adult fast isoforms fLC1, fLC2, and LC3, and occasionally traces of the slow isoforms sLC1b or sLC2. This is identical to what is found in the adult fast muscles tibialis anterior or extensor digitorum longus. It is similar to what has been observed in chicken muscle primary cultures (24–27). In primary cultures from mammalian muscles, however, most authors have reported the coexpression of embryonic and adult MLCs (18, 19). In conclusion, in the present bead culture system, myotubes after about 30 days of culture have reached a state in which al- most exclusively the adult fast isoforms of MHC as well as MLC are expressed.
Figure 3.
MLC patterns at various stages of rabbit muscle primary cultures. Two-dimensional electrophoresis of myosin extracts. All cultures grew on gelatin beads. Times indicate age of culture in days. TM, tropomyosin; TnC, troponin C; PA, parvalbumin; adult fast isoforms are fLC1, fLC2, L3; adult slow isoforms are sLC1a, sLC1b and sLC2. (a) Culture under control conditions. (b) Culture with 4 × 10−7M Ca2+ ionophore present in the medium after day 11.
Early Addition of Ca2+ Ionophore.
To study the effect of a long-term rise in [Ca2+]i, we added Ca2+ ionophore A 23187 at a final concentration of 4 × 10−7 M to part of the flasks of each preparation studied, the remainder serving as a control. The addition was started on day 11. It did not interfere with culture growth and it did not affect the spontaneous contractions of the myotubes. Table 1 shows that the ionophore concentration employed causes a marked increase in the ratio R340/380 from a control value of 0.51 to a value of 0.90 in the presence of ionophore. This increase in R340/380 corresponds appoximately to a 3- to 10-fold increase in intracellular-free Ca2+ in myotubes, as judged from a Fura-2 calibration curve. This effect is present 10 min after addition of ionophore and continues unaltered for at least 16 days.
Table 1.
Intracellular-free Ca2+ in intact myotubes (age of culture, 24 days), and ratio of CA isozyme III and II activities in myotube homogenates
| Control | With Ca2+ ionophore | |
|---|---|---|
| R340/380 (±SD) | 0.51 ± 0.02 | 0.90 ± 0.13 |
| n | 3/29 | 3/16 |
| CA III/CA II (±SD) | 0.26 ± 0.05 | 0.57 ± 0.12 |
| n | 3 | 5 |
The upper part of the table shows ratios (R340/380) of light intensities emitted by Fura-2-loaded myotubes during excitation at 340 and 380 nm, respectively, and number of preparations/number of cells studied (n). The lower part of the table shows ratios of activity of CA III over activity of CA II in myotube culture homogenates, and number of preparations (n).
The effect of increased [Ca2+]i was studied in terms of MHC and MLC pattern, the metabolic enzymes CS, LDH, and CK, and in addition by the ratio of CA isozymes CA III and CA II. Fig. 2c shows the effect on the time course of the MHC pattern. On day 22—i.e., 11 days after the addition of ionophore—the MHC electrophoresis shows MHC IId and MHC I at about equal proportions. This is not too different from the control situation (Fig. 2b), which shows the same two bands together with a significant MHC IIb band. On day 28 the fast MHC IId is much less than the slow MHC I, and on day 32 the major band present is MHC I; in addition, a minor band with the cardiac MHC Iα has appeared. This latter band is variable and is sometimes very weak or missing. It may be noted in this context that Peuker and Pette (28) have recently shown that during fast-to-slow transformation of rabbit muscles in vivo the cardiac-like MHC Iα mRNA increases several-fold so that the level of MHC Iα mRNA may be only slightly lower than that of MHC I mRNA. The appearance of its translational product in the present situation may point to a process similar to fast-to-slow transformation. We conclude that, in terms of MHC pattern, elevated Ca2+ generates a situation very similar to that produced by chronic electrostimulation-mediated fast-to-slow transformation in rabbits, resulting in the presence of adult slow MHC isoforms and the absence of fast MHC forms.
That the bead culture assumes properties of a slow type muscle under the influence of Ca2+ ionophore is confirmed by the observed MLC pattern (Fig. 3b). At all stages between day 16 and day 32 there is a predominance of the slow isoforms sLC1a and -b and sLC2. Only on day 28 there is some minor amount of fast fLC1 and fLC2 present. The most obvious feature of this slow MLC pattern is the consistent presence of sLC1a, which has the same molecular weight but a different isoelectric point compared with sLC1b. Under control conditions (Fig. 3a) sLC1a was never detectable. The MLC patterns of Fig. 3b are identical to what is obtained for the adult slow rabbit muscle soleus.
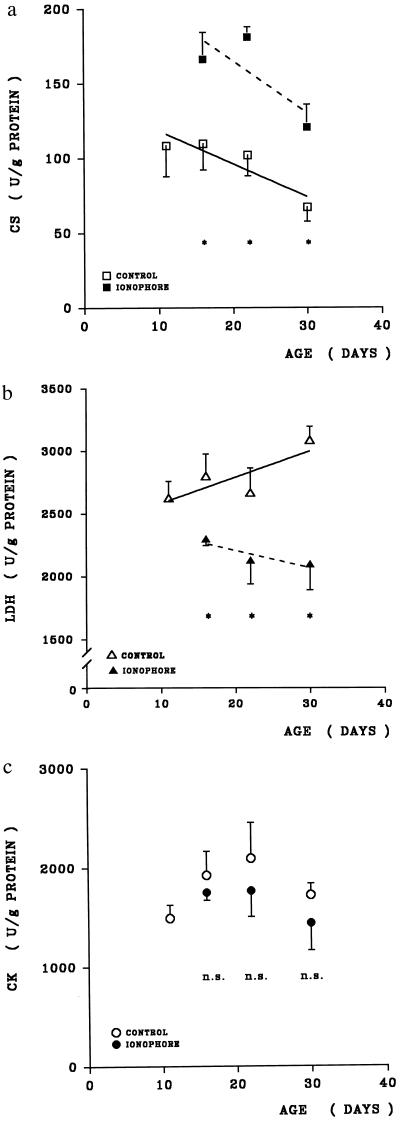
Fast and slow muscles differ with respect to their levels of the enzymes of energy metabolism (1). Fast (white) muscles show a predominance of anaerobic metabolism, slow (red) muscles of oxidative metabolism. As a marker enzyme of the former we have studied LDH and, as a marker enzyme of the latter, CS. Fig. 4 a and b shows that under the influence of elevated [Ca2+]i not only slow isomyosins but also a pattern of metabolic enzymes characteristic for slow muscles develop. In the presence of ionophore, CS is about two times the control level and LDH is about half of the control level. This is qualitatively similar to the differences observed before and after chronic electrostimulation of fast muscles (1, 29). In contrast, CK is not significantly affected by the presence of ionophore (Fig. 4c). Again, this is consistent with the observation of only a minor difference in CK levels between fast and slow muscles (30). Table 1 shows that another parameter, the ratio of CA III/CA II, exhibits an increase that is also characteristic for a fast-to-slow transition. Slow muscles such as soleus possess high activities of CA III and no CA II, while in the rabbit the reverse is true for fast muscles (12, 29). We conclude that Ca2+ ionophore A 23187, when added to the myotubes at a stage at which they still express a mixture of immature and adult isomyosins, causes them to develop within about 20 days into a state exhibiting properties of an adult slow muscle as judged by the criteria MHC, MLC, CS, LDH, and CA III/CA II.
Figure 4.
Marker enzymes of oxidative (CS) and anaerobic (LDH) metabolism and of CK in myotubes cultured for different times. Open symbols, controls; filled symbols, with ionophore A23187 present after day 11. ∗, Significance of P < 0.02 of differences between control and ionophore-treated myotubes; n.s., lack of significant differences (P > 0.1).
Reversibility of Ionophore Effect.
These experiments were performed to study whether the myotubes, once they have assumed the slow state, remain there or whether they revert to the fast state when [Ca2+]i is lowered to its normal level by withdrawing the ionophore from the culture medium. Ionophore was added to the cultures on day 8 and continued to be present until day 22, when some flasks were used for myosin and metabolic enzyme analysis. The other flasks were kept for 8 more days without ionophore, until day 30. The results for the ionophore-treated cultures and the controls are shown in Fig. 5 and Table 2. While on day 22 the controls show essentially the fast MHC IId and traces of MHCneo and MHC IIb, the 14-day ionophore treatment has led to a strong band of slow MHC I plus a strong MHC IIa and a weak MHC IId. It is interesting to note here the sequence in which MHC heavy chains replace each other when a muscle is transformed from fast to slow state (1, 31): IIb → IId → IIa → I. This probably indicates that the strong band IIa represents fibers on their way from fast IId to slow I—i.e., it indicates that these fibers are being transformed but have not yet reached the slow state. The MHC I band, on the other hand, represents fibers that have already reached this state. After 8 days of ionophore withdrawal, on day 30, the myotubes have lost all their slow MHC I and exhibit only fast MHC isoforms, mainly MHC IId and a minor band of MHC IIa; the latter probably again is indicative of the reverse transition in MHC pattern. The control myotubes on day 30 show only MHC IId.
Table 2.
Ionophore effects on marker enzymes of oxidative and anaerobic metabolism: reversibility after early ionophore addition and effect of late ionophore addition
| Age, day | Treatment | CS, units/g protein | LDH, units/g protein |
|---|---|---|---|
| 22 | Control (22 days) | 76 | 3,100 |
| 22 | Control (8 days) + ionophore (14 days) | 180 | 2,000 |
| 30 | Control (30 days) | 56 | 3,200 |
| 30 | Control (8 days) + ionophore (14 days) + control (8 days) | 65 | 3,200 |
| 22 | Control (22 days) | 110 | 2,800 |
| 30 | Control (30 days) | 63 | 2,900 |
| 30 | Control (22 days) + ionophore (8 days) | 140 | 2,200 |
The upper part of table demonstrates reversibility of effects after early addition of ionophore A23187. All measurements were performed on the same culture preparation derived from the muscles of several newborn rabbits. The lower part of table demonstrates the effect of late addition of ionophore. Again, all measurements were performed on the same culture preparation.
The upper part of Table 2 shows that also on the level of metabolic enzymes the ionophore effect can be reversed within 8 days. On day 22 specific activity of CS is 76 units/g protein in controls and 180 units/g in the ionophore-treated myotubes, LDH activity is 3,100 units/g in controls and 2,000 units/g in ionophore-exposed cultures. After 8-day withdrawal of the ionophore both enzymes are present at about equal levels in controls (CS, 56 units/g; LDH, 3,100 units/g) and in ionophore-pretreated cultures (CS, 65 units/g; LDH, 3,200 units/g).
In conclusion, withdrawal of ionophore after exposure of the myotubes for 14 days produces a rapid and marked transformation of myotubes with properties of a slow fiber into myotubes with and properties of a fast fiber. By the criteria MHC pattern CS and LDH levels this represents an example of a slow-to-fast transition.
Late Addition of Ca2+ Ionophore.
This experiment was performed to study whether a myotube that has lost embryonic and neonatal MHCs and has reached an “adult” state can be shifted from fast to slow state by the addition of ionophore. A23187 was added to the cultures on day 22, and the myotubes were exposed for 8 days until day 30. Fig. 6 shows the effects on MHC pattern. On day 22 the major MHC band is IIb, and there are also significant amounts of MHC IIa and MHC I. There is a clear effect of the ionophore, although it is not as impressive as in the reversibility experiment shown in Fig. 5: on day 30 the major isoform is MHC IIa, the “transition form;” there is also MHC I and some MHC IId. In view of the transition sequence IIb → IId → IIa → I, it is noteworthy that the large MHC IIb band of day 22 has disappeared under the influence of ionophore. Both the disappearance of IIb and the relative increase in IIa indicate a MHC rearrangement compatible with some shift from fast toward slow properties. Fig. 6 Lower shows that between day 22 and day 30 controls, on the other hand, reach stable fast properties with only MHC IId being expressed. Signs of a fast-to-slow transition are also apparent at the level of metabolic enzymes (lower half of Table 2): from a control level on day 22 of 110 units/g CS activity rises to 140 units/g after ionophore exposure for 8 days, while the control activity falls to 63 units/g. Similarly, LDH falls from a value of 2,800 units/g on day 22 to 2,200 units/g after 8 days’ treatment with ionophore, while the control remains almost unchanged with 2,900 units/g.
We conclude that late addition of Ca2+ ionophore does produce some indications of a fast-to-slow transition in terms of MHC pattern and metabolic enzymes. The effects, however, are subtle and by far not as marked as those generated by early addition or by withdrawal of ionophore.
Conclusions.
The primary rabbit muscle culture system using gelatin beads described here allows one to keep myotubes for about 30 days in standard culture medium. During such a period the myotubes appear to reach a degree of maturity in which they express only fast adult myosin as it is observed, for example, in adult fast rabbit tibialis anterior. By early addition of the Ca2+ ionophore A23187 from day 11 onwards the myotubes develop within about 20 days to assume properties of a slow muscle, such as soleus. This becomes apparent from the complete absence of all fast isoforms of MHC and MLC and their replacement by slow adult isoforms. This is accompanied by an elevated level of CS, a decreased activity of LDH, and an increased ratio of CA III/CA II. All these observations correspond to well-known differences between slow red and fast white muscles. Thus, in the presence of normal [Ca2+]i levels a fast myotube develops in culture, whereas in the presence of elevated [Ca2+]i a slow myotube grows.
This ionophore effect is rapidly reversible. When, after 14-day exposure, the ionophore is withdrawn on day 22, the myotubes have on day 30 lost the slow MHC I and possess only fast MHC. They also show nearly complete reversals of the previously elevated level of CS and the diminished level of LDH. This represents a fairly extensive slow-to-fast transformation, of a degree that even by cross-innervation experiments is difficult to achieve in vivo.
The demonstration of a transformation of a fast myotube into a slow myotube by late addition of ionophore to the culture is more difficult. Addition of ionophore on day 22 does not produce a marked increase in the slow MHC I but rather a rearrangement among the different fast MHC isoforms which points to a beginning transformation from a fast toward a slow myotube. Concomitantly, there is a moderate increase in CS and a decrease in LDH levels. Whether the smaller changes induced by late (on day 22) compared with early (on day 11) addition of ionophore are due to the more mature state of the myotubes with a diminished plasticity, or to the shorter time of exposure to ionophore (8 vs. 20 days), cannot be decided from the present data. The experiment necessary to solve this problem, keeping the cultures under control conditions for 22 days and then for another 20 days with ionophore, has not been possible so far.
Also, it is not clear why withdrawal of ionophore between day 22 and 30 causes a marked slow-to-fast transformation but addition of ionophore during the same time interval is not sufficient for a drastic fast-to-slow transformation. A possible explanation might be a slower degradation of fast MHC isoforms in the presence of ionophore A23187 than of slow MHC I in its absence. Since Silver and Etlinger (32) found no significant effect of A23187, at a concentration identical to ours, on MHC degradation in chicken muscle cultures, a more rapid degradation of slow than of fast MHCs would have to be an intrinsic property of these cells. An alternative explanation is a decreased rate of synthesis of MHCs in the presence of ionophore, and indeed, a decreased rate of synthesis of MHC (of an unspecified type) in the presence of A 23187 has been described by Silver and Etlinger (32). Further experiments are required to resolve this question.
We have shown by a variety of criteria that the level of [Ca2+]i in rabbit myotubes, when modified by addition or omission of Ca2+ ionophore A23187, determines whether these muscle cells assume properties of fast or slow muscle fibers. This suggests that the increased levels of [Ca2+]i that have been observed by Sreter et al. (4) during fast-to-slow muscle transformation by chronic electrostimulation in vivo may be a causal event, initiating the chain of processes leading to such a transformation, rather than being a secondary phenomenon. Our results do not exclude possible side effects and thus other mechanisms of action of the Ca2+ ionophore, a possibility which has to be investigated, and they do not answer the exciting question what the physiological stimulus is that mediates the long-lasting increase in [Ca2+]i described by Sreter et al. (4). However, the data reported here suggest that [Ca2+]i plays an important role in the development of a fast vs. a slow muscle and they demonstrate that the present primary muscle culture is a powerful model for further studies of the mechanism of muscle fiber transformation.
Acknowledgments
We thank Ms. Ulrike Fuhrmann for expert technical assistance. We are indebted to Dr. B. Decker (Abt. Zellbiologie und Elektroneunikopie der Medizinischen Hochschule, Hannover) for scanning electron micrographs. This study was supported by the Gesellschaft der Freunde der Medizinischen Hochschule Hannover.
ABBREVIATIONS
- [Ca2+]i
intracellular Ca2+ concentration
- MLC
myosin light chain
- MHC
myosin heavy chain
- CA
carbonic anhydrase
- CS
citrate synthase
- LDH
lactate dehydrogenase
- CK
creatine kinase
References
- 1.Pette D, Vrbová G. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- 2.Shoubridge E A, Challiss R A J, Hayes D J, Radda G K. Biochem J. 1985;232:125–131. doi: 10.1042/bj2320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henriksson J, Salmons S, Chi M M-Y, Hintz C S, Lowry O H. Am J Physiol. 1988;255:C543–C551. doi: 10.1152/ajpcell.1988.255.4.C543. [DOI] [PubMed] [Google Scholar]
- 4.Sreter F A, Lopez J R, Alamo L, Mabuchi K, Gergely J. Am J Physiol. 1987;253:C296–C300. doi: 10.1152/ajpcell.1987.253.2.C296. [DOI] [PubMed] [Google Scholar]
- 5.Green H J, Düsterhöft S, Dux L, Pette D. In: The Dynamic State of Muscle Fibers. Pette D, editor. Berlin: de Gruyter; 1990. pp. 617–627. [Google Scholar]
- 6.Robinson D G, Ehlers U, Herken R, Herrmann B, Mayer F, Schürmann F-W. Präparationsmethodik in der Elektronenmikros-kopie. Berlin: Springer; 1985. pp. 155–187. [Google Scholar]
- 7.O’Farrell P H. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 8.Carraro U, Catani C. Biochem Biophys Res Commun. 1983;116:793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- 9.Heukeshoven J, Dernick R. Electrophoresis. 1985;6:103–112. [Google Scholar]
- 10.Wetzel P, Liebner T, Gros G. FEBS Lett. 1990;267:66–70. doi: 10.1016/0014-5793(90)80289-u. [DOI] [PubMed] [Google Scholar]
- 11.Bruns W, Gros G. In: The Carbonic Anhydrases-Cellular Physiology and Molecular Genetics. Dodgson S J, Tashian R E, Gros G, Carter N D, editors. New York: Plenum; 1991. pp. 127–131. [Google Scholar]
- 12.Geers C, Krüger D, Siffert W, Schmid A, Bruns W, Gros G. Biochem J. 1992;282:165–171. doi: 10.1042/bj2820165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmeyer H U. Methods of Enzymatic Analysis. 3rd Ed. Vol. 4. Weinheim, Germany: Verlag Chemie; 1984. pp. 353–358. [Google Scholar]
- 14.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 15.Shahar A, Reuveny S. Adv Biochem Eng Biotechol. 1987;34:33–35. doi: 10.1007/BFb0000672. [DOI] [PubMed] [Google Scholar]
- 16.Nag A C, Foster J D. J Anat. 1981;132:1–18. [PMC free article] [PubMed] [Google Scholar]
- 17.Kubis H-P, Gros G. Electrophoresis. 1997;18:64–66. doi: 10.1002/elps.1150180113. [DOI] [PubMed] [Google Scholar]
- 18.Strohmann R C, Micou-Eastwood J, Glass C A, Matsuda R. Science. 1983;221:955–957. doi: 10.1126/science.6879193. [DOI] [PubMed] [Google Scholar]
- 19.Barton P J, Buckingham M E. Biochem J. 1985;231:249–261. doi: 10.1042/bj2310249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silberstein L, Webster S G, Travis M, Blau H M. Cell. 1986;46:1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- 21.Weydert A, Barton P, Harris A J, Pinset Ch, Buckingham M. Cell. 1987;49:121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- 22.Düsterhöft S, Pette D. Differentiation (Berlin) 1993;53:25–33. doi: 10.1111/j.1432-0436.1993.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 23.Naumann K, Pette D. Differentiation (Berlin) 1994;55:203–211. doi: 10.1046/j.1432-0436.1994.5530203.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein N, Holtzer H. Nature (London) 1979;280:323–325. doi: 10.1038/280323a0. [DOI] [PubMed] [Google Scholar]
- 25.Srihari T, Pette D. FEBS Lett. 1981;123:312–314. doi: 10.1016/0014-5793(81)80316-x. [DOI] [PubMed] [Google Scholar]
- 26.Stockdale F E, Baden H, Raman N. Dev Biol. 1981;82:168–171. doi: 10.1016/0012-1606(81)90438-3. [DOI] [PubMed] [Google Scholar]
- 27.Crow M T, Stockdale F E. J Cell Biol. 1985;100:1415–1422. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peuker H, Pette D. FEBS Lett. 1995;367:132–136. doi: 10.1016/0014-5793(95)00545-k. [DOI] [PubMed] [Google Scholar]
- 29.Gros G, Dodgson S J. Annu Rev Physiol. 1988;50:669–694. doi: 10.1146/annurev.ph.50.030188.003321. [DOI] [PubMed] [Google Scholar]
- 30.Lowry C V, Kimmey J S, Felder S, Chi M Y, Kaiser K K, Passonneau P N, Kirk K A, Lowry O H. J Biol Chem. 1978;253:8269–8277. [PubMed] [Google Scholar]
- 31.Staron R S, Pette D. Histochemistry. 1993;100:149–153. doi: 10.1007/BF00572901. [DOI] [PubMed] [Google Scholar]
- 32.Silver G, Etlinger J D. J Cell Biol. 1985;101:2383–2391. doi: 10.1083/jcb.101.6.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]