Abstract
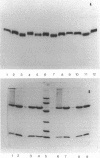
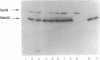
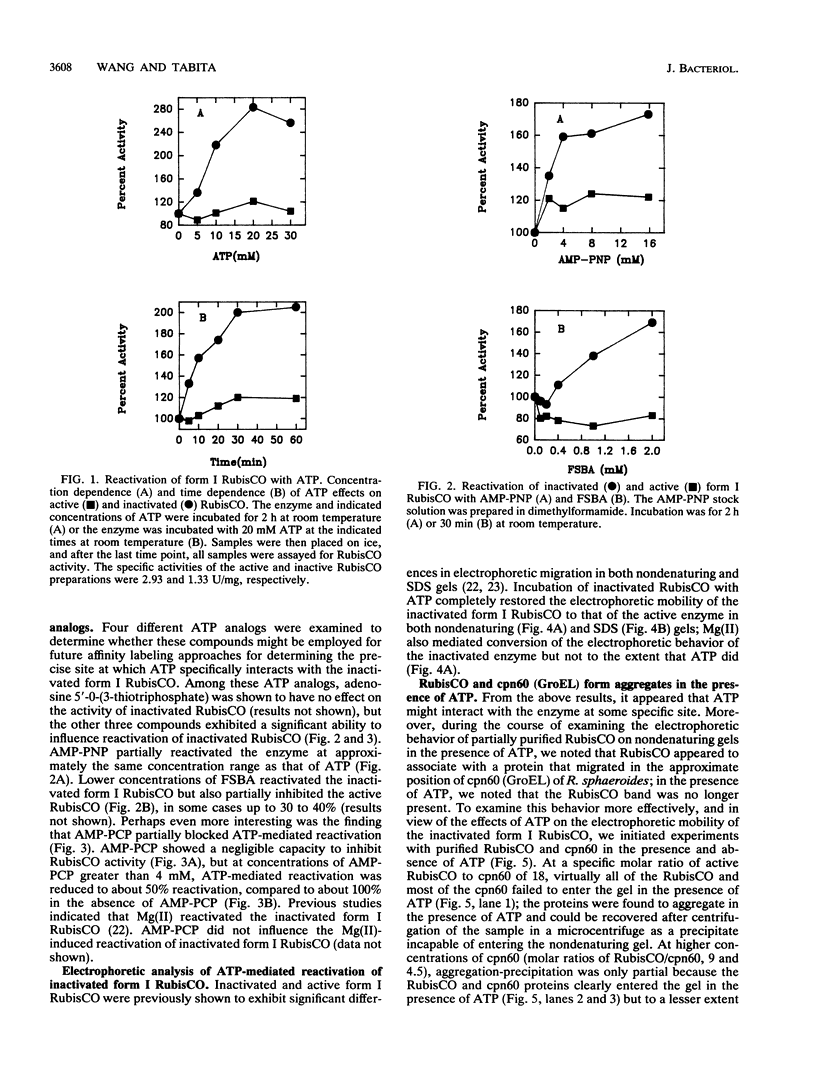
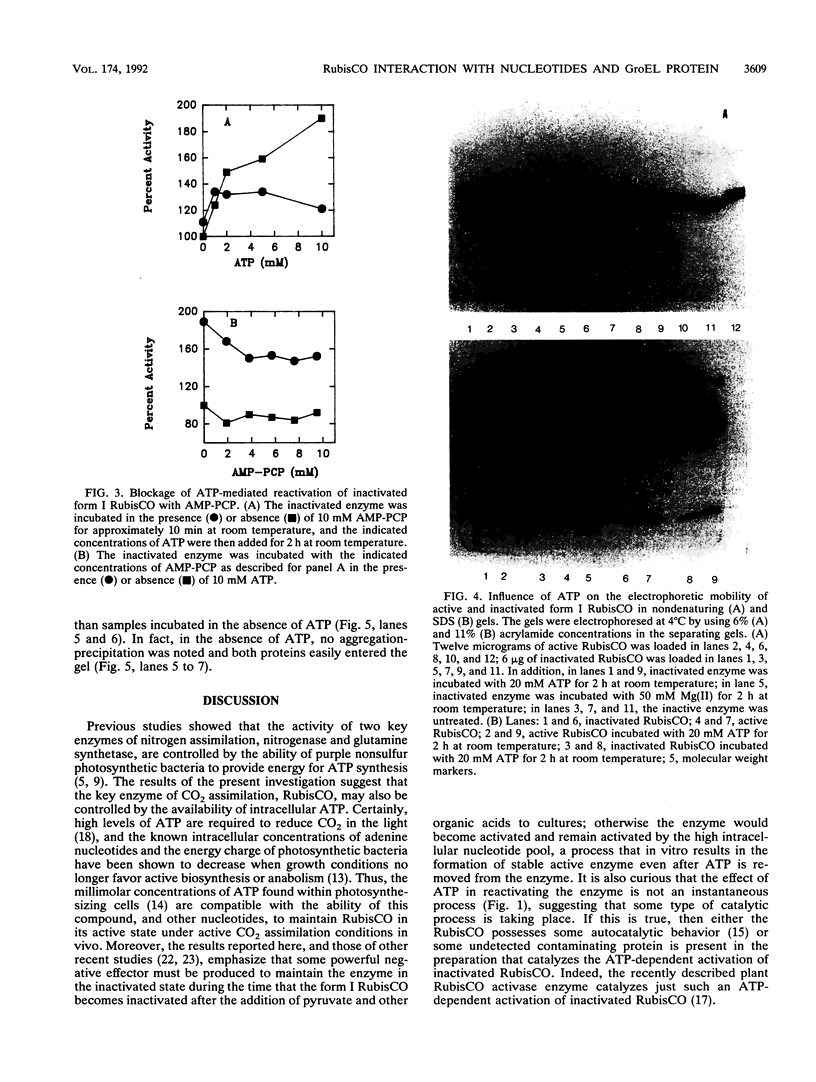
Purified inactivated form I ribulose 1,5-bisphosphate carboxylase/oxygenase (form I RubisCO) of Rhodobacter sphaeroides was activated by ATP and, to some extent, by other adenylates and nucleotides. Reactivation in the presence of ATP occurred by a time-dependent and concentration-dependent process which appeared to be irreversible. The carbamylated form of inactivated form I RubisCO was less susceptible to ATP-mediated reactivation than the uncarbamylated inactivated enzyme. In some cases, ATP analogs could mimic the reactivation process; one analog, adenylyl(beta, gamma-methylene)-diphosphonate, was found to partially block ATP-mediated reactivation but could not block reactivation induced by Mg(II). Concomitant with the recovery of enzymatic activity, the migration of the inactivated form I RubisCO on nondenaturing and sodium dodecyl sulfate gels changed from a pattern that was characteristic of inactivated enzyme to a pattern that was identical to that of the active protein. It was further found that discrete proportions of active enzyme and the chaperonin 60 protein of R. sphaeroides aggregated in the presence of ATP. The form I RubisCO is thus proposed to contain a specific ATP-binding site that may contribute to both the regulation of activity and the assembly of active enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Falcone D. L., Tabita F. R. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J Bacteriol. 1991 Mar;173(6):2099–2108. doi: 10.1128/jb.173.6.2099-2108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Falcone D. L., Tabita F. R. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Aug 5;266(22):14646–14653. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Activation of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides: probable role of the small subunit. J Bacteriol. 1979 Dec;140(3):1023–1027. doi: 10.1128/jb.140.3.1023-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. R., Tabita F. R. Cloning and expression of the chloroplast-encoded rbcL and rbcS genes from the marine diatom Cylindrotheca sp. strain N1. Plant Mol Biol. 1989 Jul;13(1):69–79. doi: 10.1007/BF00027336. [DOI] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem. 1977 Dec 1;81(2):365–371. doi: 10.1111/j.1432-1033.1977.tb11960.x. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. In vivo regulation of form I ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1987 Apr;254(1):290–303. doi: 10.1016/0003-9861(87)90105-6. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Tabita F. R. Purification of recombinant ribulose-1,5-bisphosphate carboxylase/oxygenase large subunits suitable for reconstitution and assembly of active L8S8 enzyme. Biochemistry. 1990 Oct 9;29(40):9352–9357. doi: 10.1021/bi00492a007. [DOI] [PubMed] [Google Scholar]
- Liberek K., Skowyra D., Zylicz M., Johnson C., Georgopoulos C. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J Biol Chem. 1991 Aug 5;266(22):14491–14496. [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools in growing Chromatium strain D. J Bacteriol. 1971 Nov;108(2):954–956. doi: 10.1128/jb.108.2.954-956.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlesky K. C., Tabita F. R. Purification and characterization of the chaperonin 10 and chaperonin 60 proteins from Rhodobacter sphaeroides. Biochemistry. 1991 Aug 20;30(33):8181–8186. doi: 10.1021/bi00247a013. [DOI] [PubMed] [Google Scholar]
- Tesson F., Prip-Buus C., Lemoine A., Pegorier J. P., Parini A. Subcellular distribution of imidazoline-guanidinium-receptive sites in human and rabbit liver. Major localization to the mitochondrial outer membrane. J Biol Chem. 1991 Jan 5;266(1):155–160. [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Wang X., Tabita F. R. Interaction between ribulose 1,5-bisphosphate carboxylase/oxygenase activity and the ammonia assimilatory system of Rhodobacter sphaeroides. J Bacteriol. 1992 Jun;174(11):3601–3606. doi: 10.1128/jb.174.11.3601-3606.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tabita F. R. Reversible inactivation and characterization of purified inactivated form I ribulose 1,5-bisphosphate carboxylase/oxygenase of Rhodobacter sphaeroides. J Bacteriol. 1992 Jun;174(11):3593–3600. doi: 10.1128/jb.174.11.3593-3600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]