Abstract
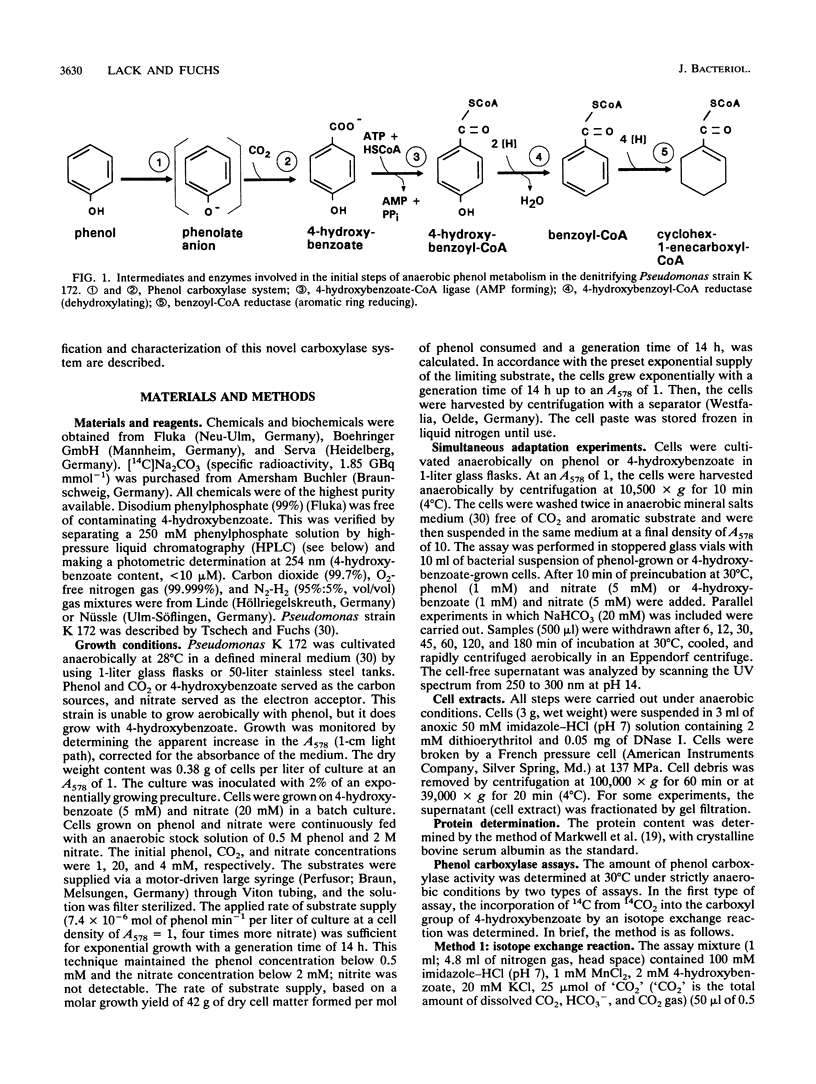
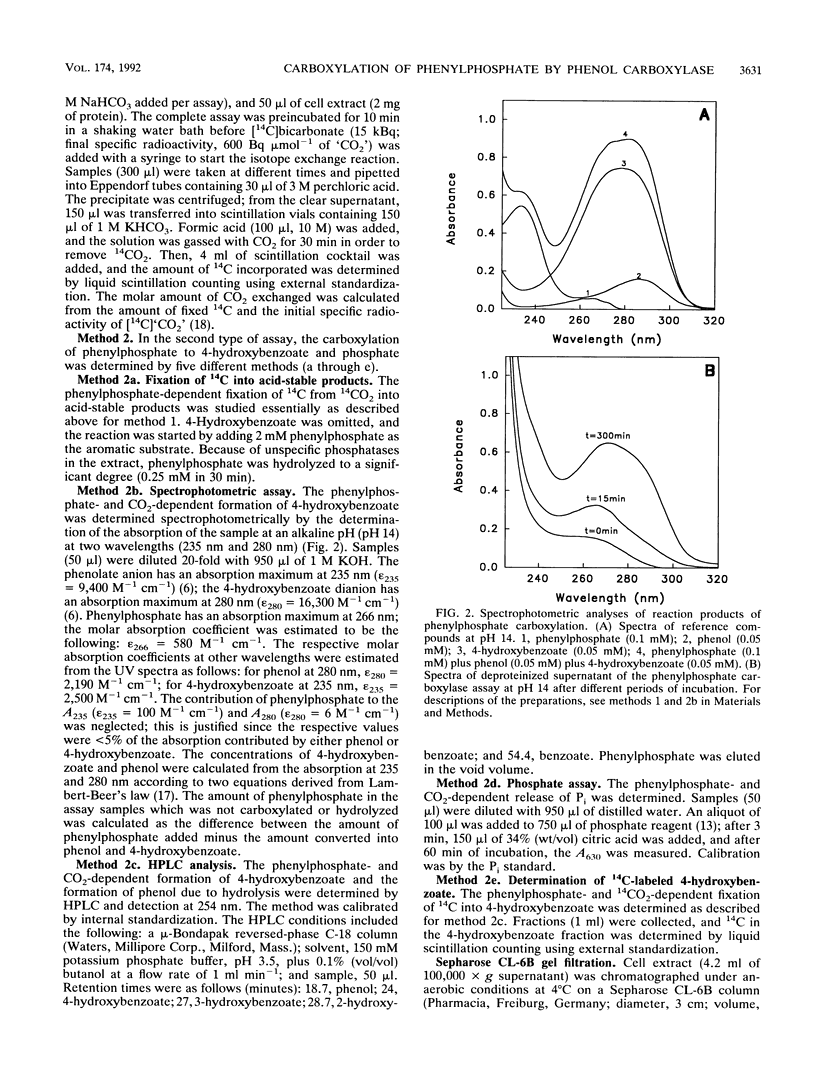
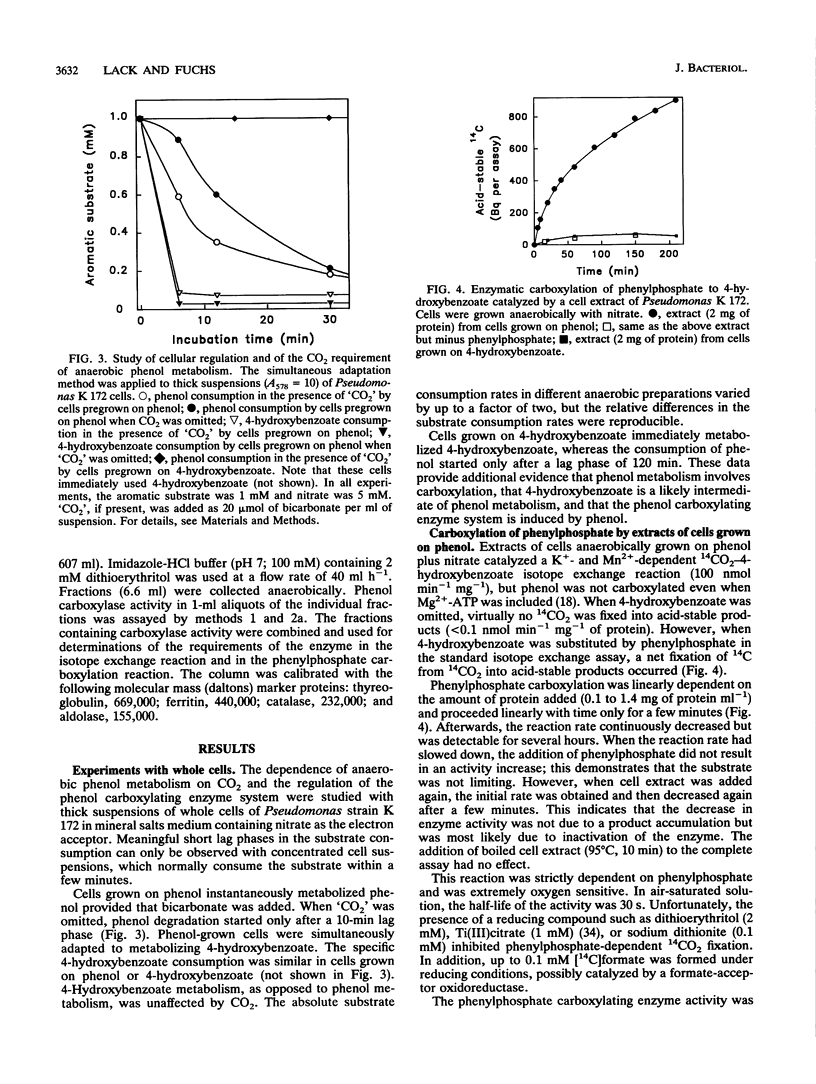
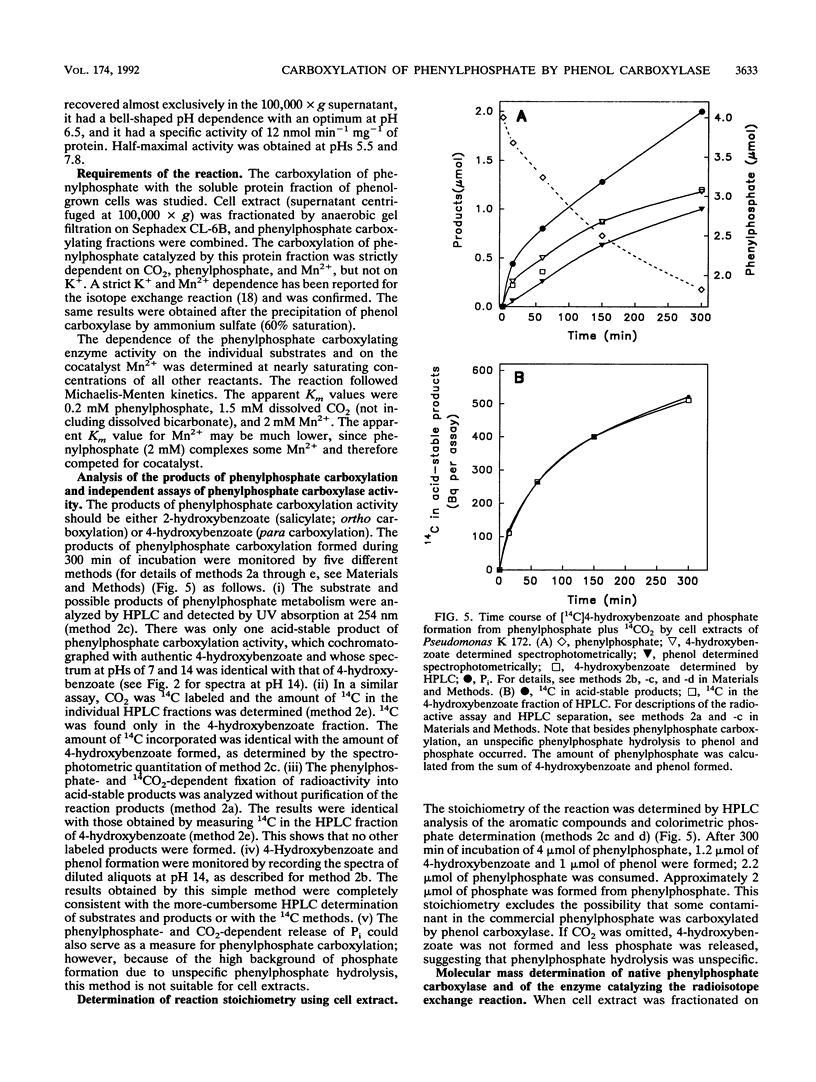
Several lines of evidence indicate that the first step in the anaerobic metabolism of phenol is phenol carboxylation to 4-hydroxybenzoate; this reaction is considered a biological Kolbe-Schmitt carboxylation. A phenol carboxylase system was characterized by using a denitrifying Pseudomonas strain, K 172, which catalyzes an isotope exchange between 14CO2 and the carboxyl group of 4-hydroxybenzoate. The enzymatic isotope exchange activity (100 nmol min-1 mg-1 of protein) requires Mn2+ and K+. We show that this system also catalyzes the carboxylation of phenylphosphate (the phosphoric acid monophenyl ester) to 4-hydroxybenzoate and phosphate. The specific activity of phenylphosphate carboxylation at the optimal pH of 6.5 is 12 nmol of CO2 fixed min-1 mg-1 of protein. Phenylphosphate cannot be replaced by Mg(2+)-ATP and phenol. The carboxylase activity requires Mn2+ but, in contrast to the isotope exchange activity, does not require K+. The apparent Km values are 1.5 mM dissolved CO2 and 0.2 mM phenylphosphate. Several convenient assays for phenylophosphate carboxylation are described. The isotope exchange reaction and the net carboxylation reaction are catalyzed by the same oxygen-sensitive enzyme, which has a half-life in an air-saturated solution of less than 1 min. Both activities cochromatographed with a protein with a Mr of 280,000, and both activities were induced only after anaerobic growth on phenol. The carboxylation of phenylphosphate suggests that phenylphosphate itself is the physiological CO2 acceptor molecular of this novel CO2 fixation reaction. Alternatively, phenylphosphate could simulate the unknown natural precursor. It is suggested that the formation of an enzyme-bound phenolate anion from the activated phenolic compound is the rate-determining step in the carboxylation reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisaillon J. G., Lépine F., Beaudet R., Sylvestre M. Carboxylation of o-cresol by an anaerobic consortium under methanogenic conditions. Appl Environ Microbiol. 1991 Aug;57(8):2131–2134. doi: 10.1128/aem.57.8.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Townsend G. T., Chapman P. J. Anaerobic transformation of phenol to benzoate via para-carboxylation: use of fluorinated analogues to elucidate the mechanism of transformation. Biochem Biophys Res Commun. 1989 Aug 15;162(3):945–951. doi: 10.1016/0006-291x(89)90764-x. [DOI] [PubMed] [Google Scholar]
- Glöckler R., Tschech A., Fuchs G. Reductive dehydroxylation of 4-hydroxybenzoyl-CoA to benzoyl-CoA in a denitrifying, phenol-degrading Pseudomonas species. FEBS Lett. 1989 Jul 17;251(1-2):237–240. doi: 10.1016/0014-5793(89)81461-9. [DOI] [PubMed] [Google Scholar]
- Koch J., Fuchs G. Enzymatic reduction of benzoyl-CoA to alicyclic compounds, a key reaction in anaerobic aromatic metabolism. Eur J Biochem. 1992 Apr 1;205(1):195–202. doi: 10.1111/j.1432-1033.1992.tb16768.x. [DOI] [PubMed] [Google Scholar]
- Lack A., Tommasi I., Aresta M., Fuchs G. Catalytic properties of phenol carboxylase. In vitro study of CO2: 4-hydroxybenzoate isotope exchange reaction. Eur J Biochem. 1991 Apr 23;197(2):473–479. doi: 10.1111/j.1432-1033.1991.tb15934.x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Merkel S. M., Eberhard A. E., Gibson J., Harwood C. S. Involvement of coenzyme A thioesters in anaerobic metabolism of 4-hydroxybenzoate by Rhodopseudomonas palustris. J Bacteriol. 1989 Jan;171(1):1–7. doi: 10.1128/jb.171.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platen H., Schink B. Anaerobic degradation of acetone and higher ketones via carboxylation by newly isolated denitrifying bacteria. J Gen Microbiol. 1989 Apr;135(4):883–891. doi: 10.1099/00221287-135-4-883. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Fedorak P. M., Hrudey S. E. CO(2) Incorporation and 4-Hydroxy-2-Methylbenzoic Acid Formation during Anaerobic Metabolism of m-Cresol by a Methanogenic Consortium. Appl Environ Microbiol. 1990 Feb;56(2):472–478. doi: 10.1128/aem.56.2.472-478.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi A., Tschech A., Fuchs G. Anaerobic degradation of cresols by denitrifying bacteria. Arch Microbiol. 1991;155(3):238–248. doi: 10.1007/BF00252207. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Metabolism of phenolic acids by the rat intestinal microflora. Acta Pharmacol Toxicol (Copenh) 1968;26(2):189–205. doi: 10.1111/j.1600-0773.1968.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Schnell S., Bak F., Pfennig N. Anaerobic degradation of aniline and dihydroxybenzenes by newly isolated sulfate-reducing bacteria and description of Desulfobacterium anilini. Arch Microbiol. 1989;152(6):556–563. doi: 10.1007/BF00425486. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]


