Abstract
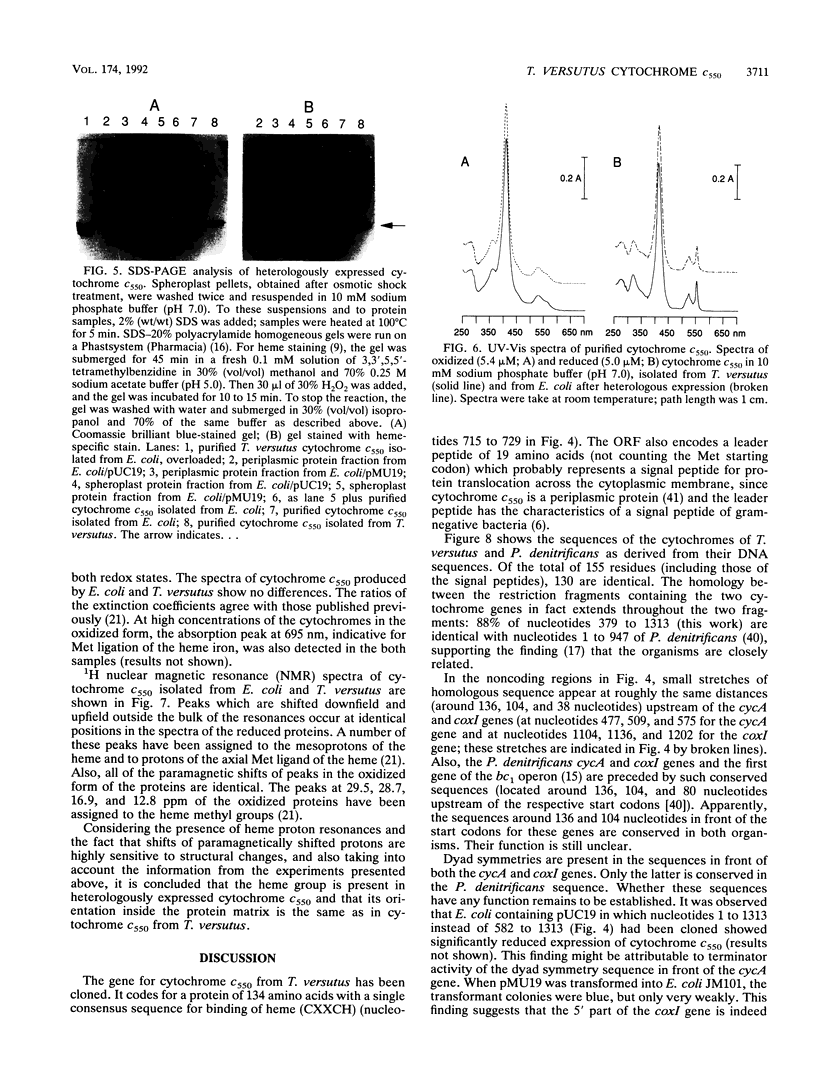
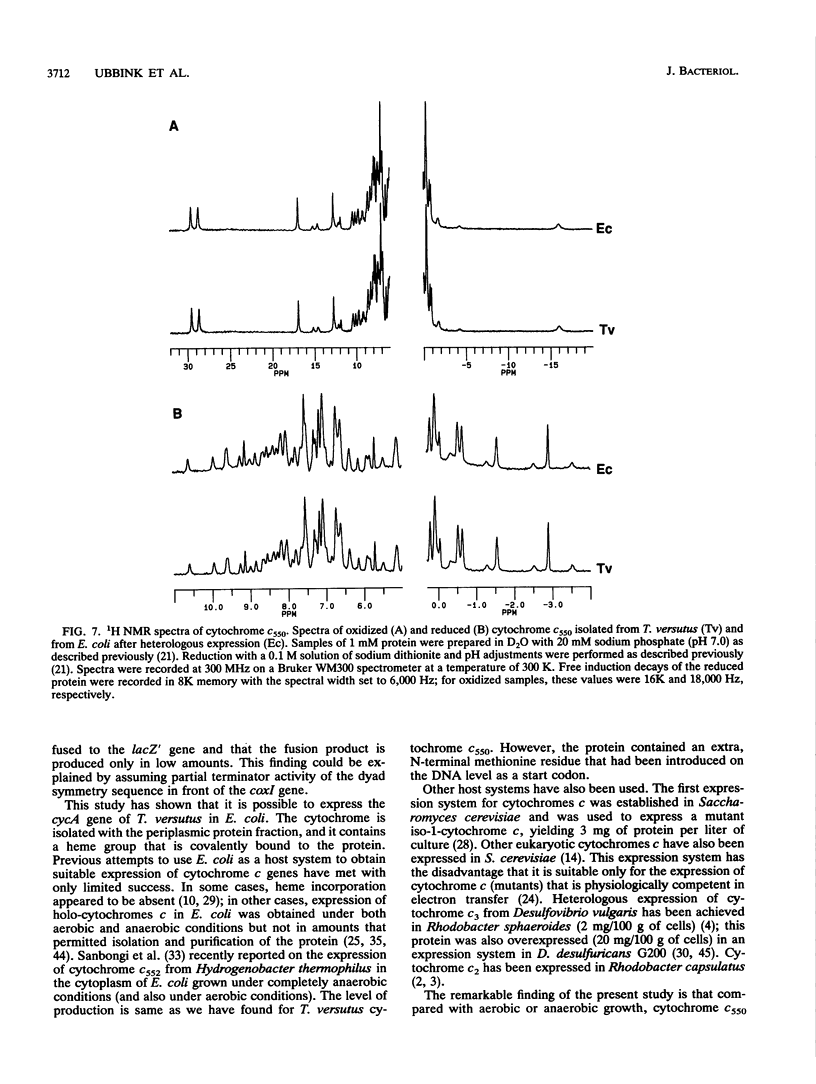
The gene coding for cytochrome c550 from Thiobacillus versutus, cycA, has been cloned and sequenced. It codes for a protein of 134 amino acids plus a 19-amino-acid-long signal peptide. Both coding and noncoding DNA sequences of the clone are homologous to the Paracoccus denitrificans DNA sequence. An expression vector was constructed by cloning the cycA gene directly behind the lac promoter of pUC. The cycA gene was expressed in Escherichia coli under semianaerobic conditions, and mature holo-cytochrome c550 was isolated with the periplasmic soluble protein fraction. Under both aerobic and anaerobic conditions, significantly less cytochrome c550 was produced. The heterologously expressed cytochrome c550 was isolated and purified to better than 95% purity and was compared with cytochrome c550 isolated and purified from T. versutus. No structural differences could be detected by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis UV-visible light spectroscopy, and 1H nuclear magnetic resonance spectroscopy, indicating that E. coli produces the cytochrome and attaches the heme correctly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M. S., Cusanovich M. A. Lysines in the amino-terminal alpha-helix are important to the stability of Rhodobacter capsulatus cytochrome c2. Biochemistry. 1991 Sep 24;30(38):9238–9241. doi: 10.1021/bi00102a015. [DOI] [PubMed] [Google Scholar]
- Caffrey M. S., Daldal F., Holden H. M., Cusanovich M. A. Importance of a conserved hydrogen-bonding network in cytochromes c to their redox potentials and stabilities. Biochemistry. 1991 Apr 30;30(17):4119–4125. doi: 10.1021/bi00231a002. [DOI] [PubMed] [Google Scholar]
- Cannac V., Caffrey M. S., Voordouw G., Cusanovich M. A. Expression of the gene encoding cytochrome c3 from the sulfate-reducing bacterium Desulfovibrio vulgaris in the purple photosynthetic bacterium Rhodobacter sphaeroides. Arch Biochem Biophys. 1991 May 1;286(2):629–632. doi: 10.1016/0003-9861(91)90091-v. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grisshammer R., Oeckl C., Michel H. Expression in Escherichia coli of c-type cytochrome genes from Rhodopseudomonas viridis. Biochim Biophys Acta. 1991 Feb 16;1088(2):183–190. doi: 10.1016/0167-4781(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Koshy T. I., Luntz T. L., Schejter A., Margoliash E. Changing the invariant proline-30 of rat and Drosophila melanogaster cytochromes c to alanine or valine destabilizes the heme crevice more than the overall conformation. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8697–8701. doi: 10.1073/pnas.87.22.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski B., Ludwig B. The genes of the Paracoccus denitrificans bc1 complex. Nucleotide sequence and homologies between bacterial and mitochondrial subunits. J Biol Chem. 1987 Oct 5;262(28):13805–13811. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Stahl D. A., Olsen G. J., Heller D. J., Pace N. R. Phylogenetic analysis of the genera Thiobacillus and Thiomicrospira by 5S rRNA sequences. J Bacteriol. 1985 Jul;163(1):75–81. doi: 10.1128/jb.163.1.75-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen A., Canters G. W. pH-dependent redox activity and fluxionality of the copper site in amicyanin from Thiobacillus yersutus as studied by 300- and 600- MHz 1H NMR. J Biol Chem. 1990 Feb 15;265(5):2768–2774. [PubMed] [Google Scholar]
- Lommen A., Canters G. W., van Beeumen J. A 1H-NMR study on the blue copper protein amicyanin from Thiobacillus versutus. Resonance identifications, structural rearrangements and determination of the electron self-exchange rate constant. Eur J Biochem. 1988 Sep 1;176(1):213–223. doi: 10.1111/j.1432-1033.1988.tb14271.x. [DOI] [PubMed] [Google Scholar]
- Lommen A., Pandya K. I., Koningsberger D. C., Canters G. W. EXAFS analysis of the pH dependence of the blue-copper site in amicyanin from Thiobacillus versutus. Biochim Biophys Acta. 1991 Feb 15;1076(3):439–447. doi: 10.1016/0167-4838(91)90489-m. [DOI] [PubMed] [Google Scholar]
- Lommen A., Ratsma A., Bijlsma N., Canters G. W., van Wielink J. E., Frank J., van Beeumen J. Isolation and characterization of cytochrome c550 from the methylamine-oxidizing electron-transport chain of Thiobacillus versutus. Eur J Biochem. 1990 Sep 24;192(3):653–661. doi: 10.1111/j.1432-1033.1990.tb19272.x. [DOI] [PubMed] [Google Scholar]
- Lommen A., Wijmenga S., Hilbers C. W., Canters G. W. Assignment of the 600-MHz 1H-NMR spectrum of amicyanin from Thiobacillus versutus by two-dimensional NMR methods provides information on secondary structure. Eur J Biochem. 1991 Nov 1;201(3):695–702. doi: 10.1111/j.1432-1033.1991.tb16330.x. [DOI] [PubMed] [Google Scholar]
- McEwan A. G., Kaplan S., Donohue T. J. Synthesis of Rhodobacter sphaeroides cytochrome c2 in Escherichia coli. FEMS Microbiol Lett. 1989 Jun;50(3):253–258. doi: 10.1016/0378-1097(89)90427-8. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pielak G. J., Mauk A. G., Smith M. Site-directed mutagenesis of cytochrome c shows that an invariant Phe is not essential for function. Nature. 1985 Jan 10;313(5998):152–154. doi: 10.1038/313152a0. [DOI] [PubMed] [Google Scholar]
- Pollock W. B., Chemerika P. J., Forrest M. E., Beatty J. T., Voordouw G. Expression of the gene encoding cytochrome c3 from Desulfovibrio vulgaris (Hildenborough) in Escherichia coli: export and processing of the apoprotein. J Gen Microbiol. 1989 Aug;135(8):2319–2328. doi: 10.1099/00221287-135-8-2319. [DOI] [PubMed] [Google Scholar]
- Pollock W. B., Loutfi M., Bruschi M., Rapp-Giles B. J., Wall J. D., Voordouw G. Cloning, sequencing, and expression of the gene encoding the high-molecular-weight cytochrome c from Desulfovibrio vulgaris Hildenborough. J Bacteriol. 1991 Jan;173(1):220–228. doi: 10.1128/jb.173.1.220-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitio M., Pispa J. M., Metso T., Saraste M. Are there isoenzymes of cytochrome c oxidase in Paracoccus denitrificans? FEBS Lett. 1990 Feb 26;261(2):431–435. doi: 10.1016/0014-5793(90)80609-m. [DOI] [PubMed] [Google Scholar]
- Sanbongi Y., Yang J. H., Igarashi Y., Kodama T. Cloning, nucleotide sequence and expression of the cytochrome c-552 gene from Hydrogenobacter thermophilus. Eur J Biochem. 1991 May 23;198(1):7–12. doi: 10.1111/j.1432-1033.1991.tb15979.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self S. J., Hunter C. N., Leatherbarrow R. J. Molecular cloning, sequencing and expression of cytochrome c2 from Rhodospirillum rubrum. Biochem J. 1990 Jan 15;265(2):599–604. doi: 10.1042/bj2650599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Ubbink M., van Kleef M. A., Kleinjan D. J., Hoitink C. W., Huitema F., Beintema J. J., Duine J. A., Canters G. W. Cloning, sequencing and expression studies of the genes encoding amicyanin and the beta-subunit of methylamine dehydrogenase from Thiobacillus versutus. Eur J Biochem. 1991 Dec 18;202(3):1003–1012. doi: 10.1111/j.1432-1033.1991.tb16462.x. [DOI] [PubMed] [Google Scholar]
- Van Beeumen J., Van Bun S., Canters G. W., Lommen A., Chothia C. The structural homology of amicyanin from Thiobacillus versutus to plant plastocyanins. J Biol Chem. 1991 Mar 15;266(8):4869–4877. [PubMed] [Google Scholar]
- Van Spanning R. J., Wansell C., Harms N., Oltmann L. F., Stouthamer A. H. Mutagenesis of the gene encoding cytochrome c550 of Paracoccus denitrificans and analysis of the resultant physiological effects. J Bacteriol. 1990 Feb;172(2):986–996. doi: 10.1128/jb.172.2.986-996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellieux F. M., Frank J., Swarte M. B., Groendijk H., Duine J. A., Drenth J., Hol W. G. Purification, crystallization and preliminary X-ray investigation of quinoprotein methylamine dehydrogenase from Thiobacillus versutus. Eur J Biochem. 1986 Jan 15;154(2):383–386. doi: 10.1111/j.1432-1033.1986.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Vellieux F. M., Huitema F., Groendijk H., Kalk K. H., Jzn J. F., Jongejan J. A., Duine J. A., Petratos K., Drenth J., Hol W. G. Structure of quinoprotein methylamine dehydrogenase at 2.25 A resolution. EMBO J. 1989 Aug;8(8):2171–2178. doi: 10.1002/j.1460-2075.1989.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., Pollock W. B., Bruschi M., Guerlesquin F., Rapp-Giles B. J., Wall J. D. Functional expression of Desulfovibrio vulgaris Hildenborough cytochrome c3 in Desulfovibrio desulfuricans G200 after conjugational gene transfer from Escherichia coli. J Bacteriol. 1990 Oct;172(10):6122–6126. doi: 10.1128/jb.172.10.6122-6126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houwelingen T., Canters G. W., Stobbelaar G., Duine J. A., Frank J., Jr, Tsugita A. Isolation and characterization of a blue copper protein from Thiobacillus versutus. Eur J Biochem. 1985 Nov 15;153(1):75–80. doi: 10.1111/j.1432-1033.1985.tb09268.x. [DOI] [PubMed] [Google Scholar]
- von Wachenfeldt C., Hederstedt L. Bacillus subtilis holo-cytochrome c-550 can be synthesised in aerobic Escherichia coli. FEBS Lett. 1990 Sep 17;270(1-2):147–151. doi: 10.1016/0014-5793(90)81255-m. [DOI] [PubMed] [Google Scholar]