Abstract
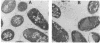
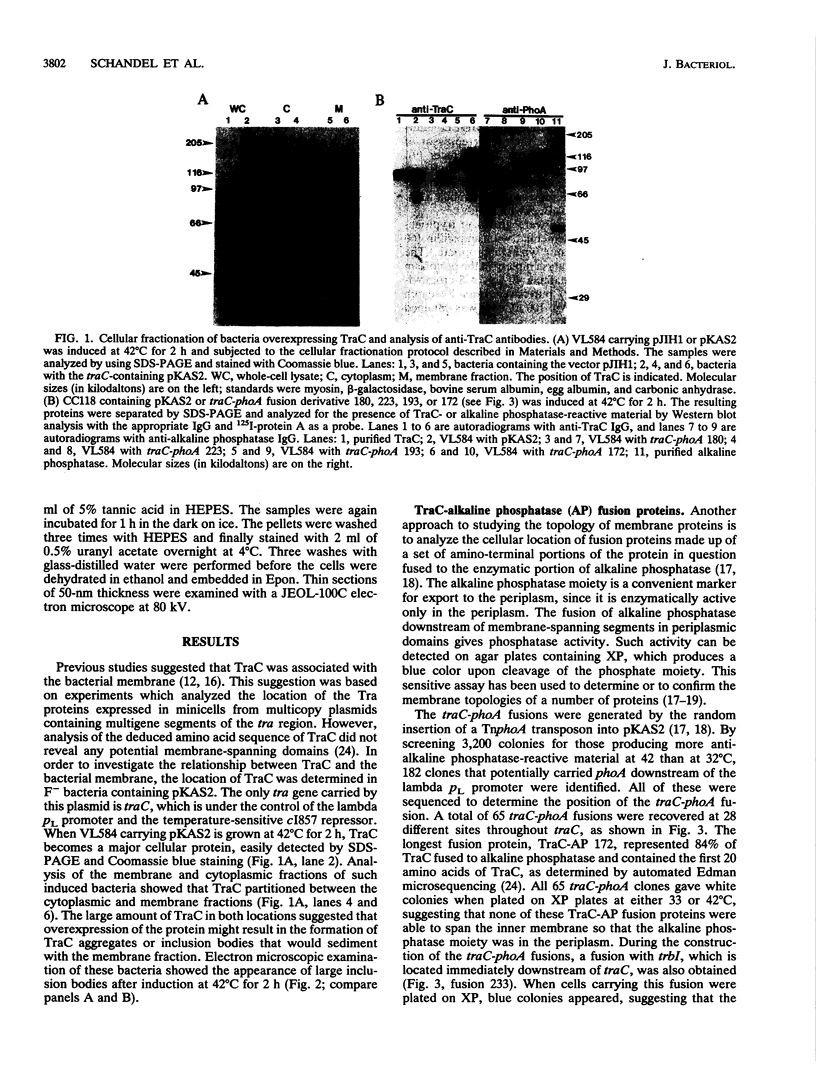
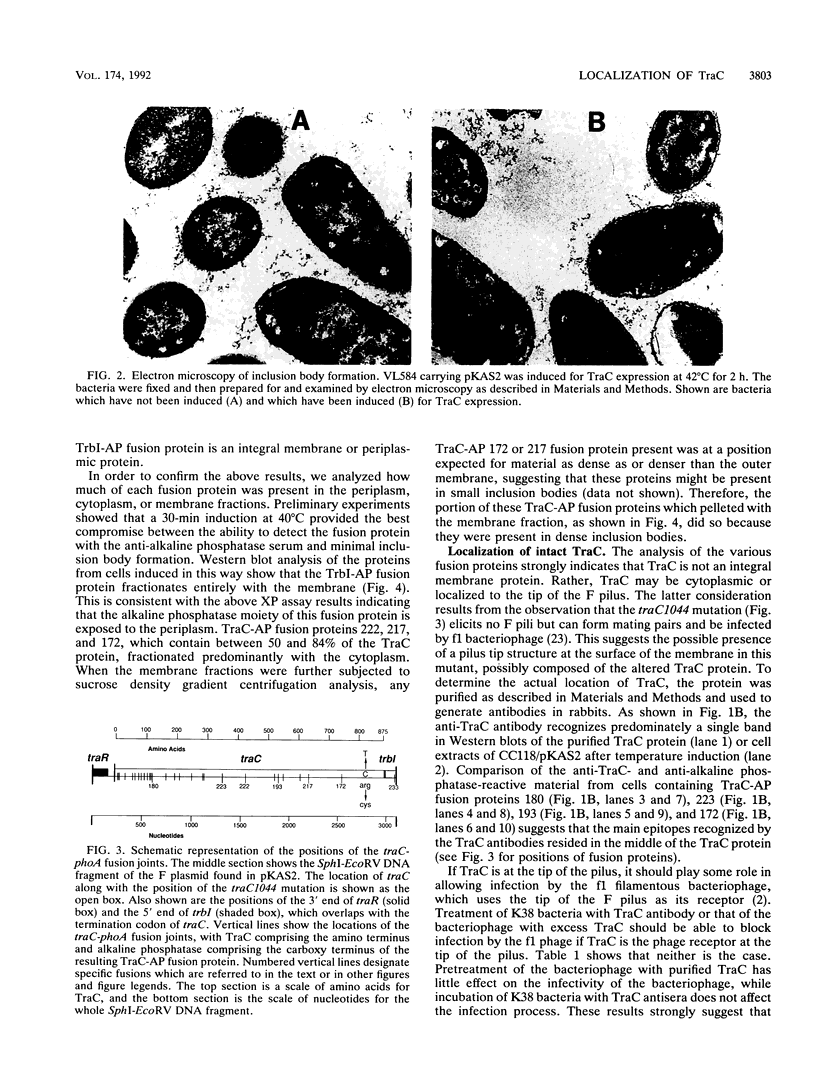
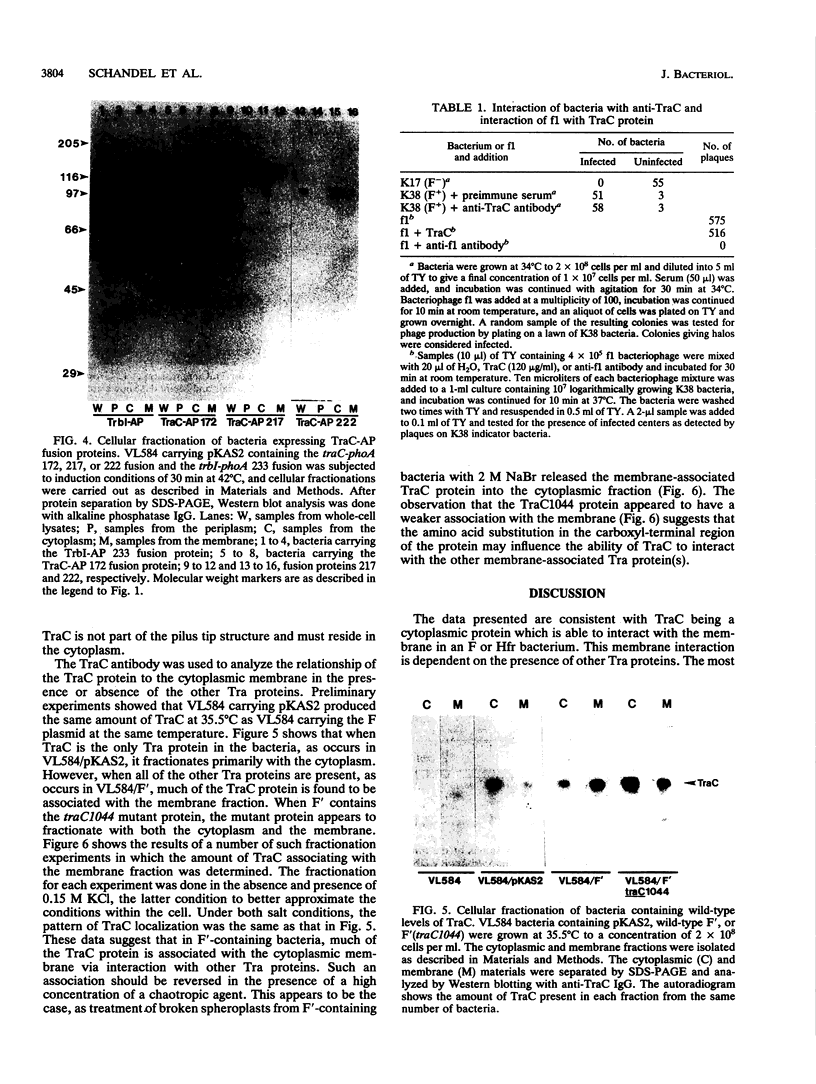
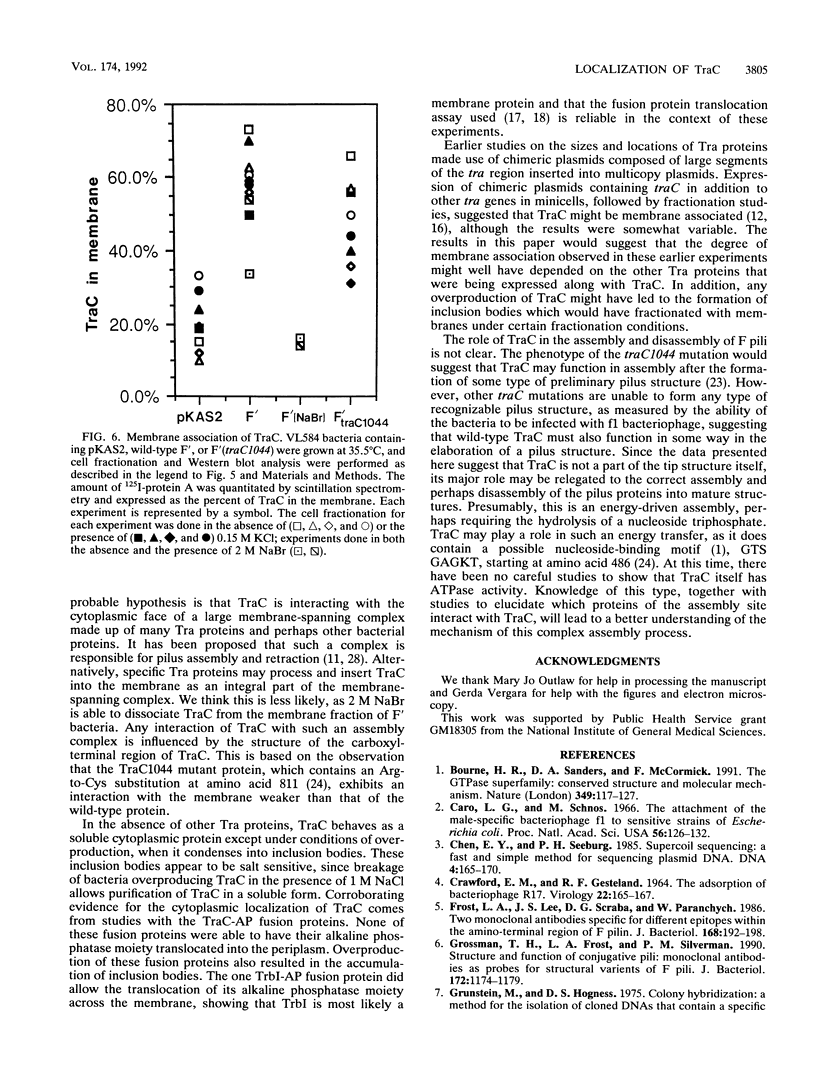
TraC is one of the proteins encoded by the F transfer region of the F conjugative plasmid which is required for the assembly of F pilin into the mature F pilus structure. Overproduction of this protein from the plasmid pKAS2, which carries only traC, resulted in the formation of inclusion bodies from which soluble TraC was purified. When small amounts of TraC were produced from pKAS2, the protein was localized to the cytoplasm by using anti-TraC antibodies. Similar analysis of a set of TraC-alkaline phosphatase fusion proteins localized all of these fusion proteins to the cytoplasm. However, when TraC was expressed from the F plasmid, much of it appeared associated with the bacterial membrane fraction. Under these conditions, TraC does not appear to be part of the tip of the F pilus, as neither anti-TraC antibodies nor purified TraC had any effect on the infection of F-containing bacteria by the filamentous bacteriophage f1. These data suggest that TraC is normally associated with the membrane through interactions with other proteins specified by the tra region. This interaction may be via the carboxyl-terminal region of the TraC protein, as a mutant TraC protein containing an Arg-Cys substitution at amino acid 811 exhibits an interaction with the membrane weaker than that of the wild-type protein in the presence of the other Tra proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Lee J. S., Scraba D. G., Paranchych W. Two monoclonal antibodies specific for different epitopes within the amino-terminal region of F pilin. J Bacteriol. 1986 Oct;168(1):192–198. doi: 10.1128/jb.168.1.192-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T. H., Frost L. S., Silverman P. M. Structure and function of conjugative pili: monoclonal antibodies as probes for structural variants of F pili. J Bacteriol. 1990 Mar;172(3):1174–1179. doi: 10.1128/jb.172.3.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T. H., Frost L. S., Silverman P. M. Structure and function of conjugative pili: monoclonal antibodies as probes for structural variants of F pili. J Bacteriol. 1990 Mar;172(3):1174–1179. doi: 10.1128/jb.172.3.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham L. M., Firth N., Skurray R. Nucleotide sequence of the F plasmid transfer gene, traH: identification of a new gene and a promoter within the transfer operon. Gene. 1989 Jan 30;75(1):157–165. doi: 10.1016/0378-1119(89)90392-2. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Webster R. E. Morphogenesis of f1 filamentous bacteriophage. Increased expression of gene I inhibits bacterial growth. J Mol Biol. 1986 Apr 5;188(3):403–413. doi: 10.1016/0022-2836(86)90164-6. [DOI] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Zinder N. D. The genetic map of the filamentous bacteriophage f1. Virology. 1972 Jul;49(1):45–60. doi: 10.1016/s0042-6822(72)80006-0. [DOI] [PubMed] [Google Scholar]
- Maneewannakul S., Maneewannakul K., Ippen-Ihler K. Characterization of trbC, a new F plasmid tra operon gene that is essential to conjugative transfer. J Bacteriol. 1991 Jun;173(12):3872–3878. doi: 10.1128/jb.173.12.3872-3878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Morelli G., Achtman M. traG protein of the F sex factor of Escherichia coli K-12 and its role in conjugation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7487–7491. doi: 10.1073/pnas.78.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Boyd D., Beckwith J. Molecular genetic analysis of membrane protein topology. Trends Genet. 1988 Aug;4(8):223–226. doi: 10.1016/0168-9525(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Rasched I., Oberer E. Ff coliphages: structural and functional relationships. Microbiol Rev. 1986 Dec;50(4):401–427. doi: 10.1128/mr.50.4.401-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel K. A., Maneewannakul S., Ippen-Ihler K., Webster R. E. A traC mutant that retains sensitivity to f1 bacteriophage but lacks F pili. J Bacteriol. 1987 Jul;169(7):3151–3159. doi: 10.1128/jb.169.7.3151-3159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel K. A., Maneewannakul S., Vonder Haar R. A., Ippen-Ihler K., Webster R. E. Nucleotide sequence of the F plasmid gene, traC, and identification of its product. Gene. 1990 Nov 30;96(1):137–140. doi: 10.1016/0378-1119(90)90354-t. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Wu J. H., Kathir P., Ippen-Ihler K. The product of the F plasmid transfer operon gene, traF, is a periplasmic protein. J Bacteriol. 1988 Aug;170(8):3633–3639. doi: 10.1128/jb.170.8.3633-3639.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Bacteriophage f1 gene II and X proteins. Isolation and characterization of the products of two overlapping genes. J Biol Chem. 1981 Nov 10;256(21):11259–11265. [PubMed] [Google Scholar]