Abstract
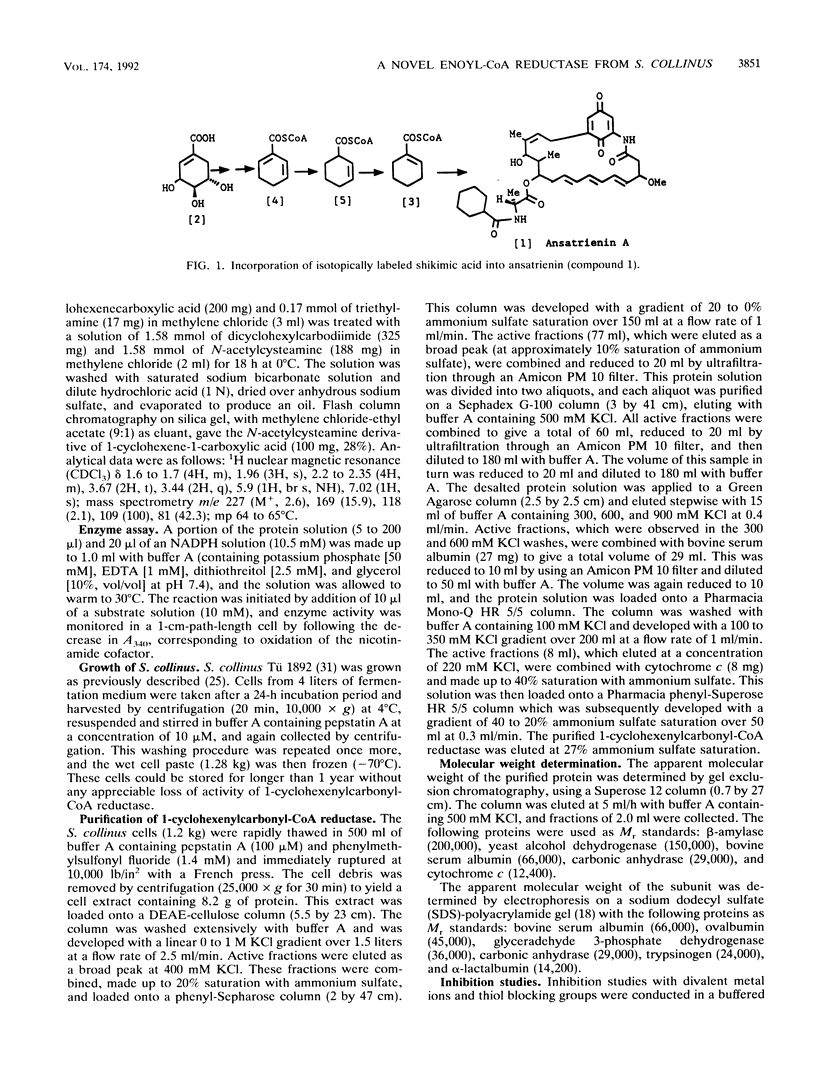
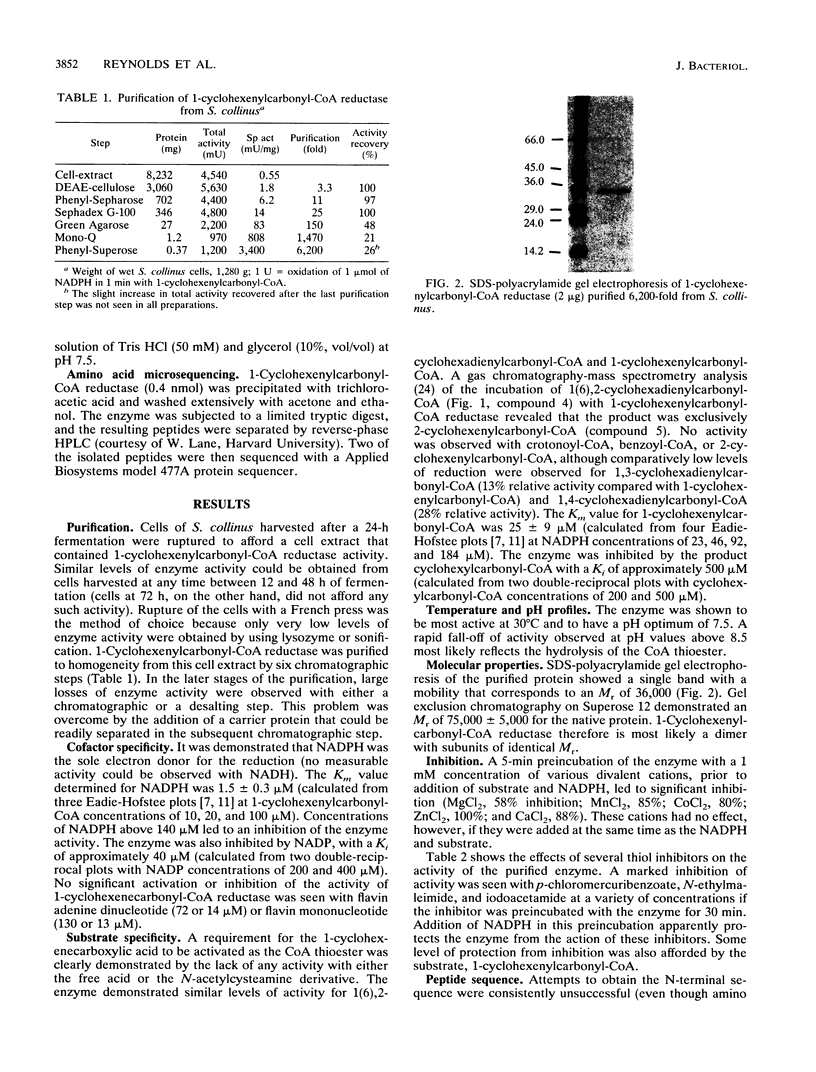
A novel NADPH-dependent enoyl reductase, catalyzing the conversion of 1-cyclohexenylcarbonyl coenzyme A (1-cyclohexenylcarbonyl-CoA) to cyclohexylcarbonyl-CoA, was purified to homogeneity from Streptomyces collinus. This enzyme, a dimer with subunits of identical M(r) (36,000), exhibits a Km of 1.5 +/- 0.3 microM for NADPH and 25 +/- 3 microM for 1-cyclohexenylcarbonyl-CoA. It has a pH optimum of 7.5, is most active at 30 degrees C, and is inhibited by both divalent cations and thiol reagents. Two internal peptide sequences were obtained. Ansatrienin A (an antibiotic produced by S. collinus) contains a cyclohexanecarboxylic acid moiety, and it is suggested that the 1-cyclohexenylcarbonyl-CoA reductase described herein catalyzes the final reductive step in the conversion of shikimic acid into this moiety.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson V. E., Hammes G. G. Stereochemistry of the reactions catalyzed by chicken liver fatty acid synthase. Biochemistry. 1984 Apr 24;23(9):2088–2094. doi: 10.1021/bi00304a033. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiang C. F. Purification of rat liver microsomal trans-2-enoyl-CoA reductase. Prep Biochem. 1987;17(4):315–325. doi: 10.1080/00327488708062498. [DOI] [PubMed] [Google Scholar]
- Cvetanović M., Moreno de la Garza M., Dommes V., Kunau W. H. Purification and characterization of 2-enoyl-CoA reductase from bovine liver. Biochem J. 1985 Apr 1;227(1):49–56. doi: 10.1042/bj2270049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J. C., Schulz H. Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol. 1981;71(Pt 100):390–398. doi: 10.1016/0076-6879(81)71049-8. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Inui H., Miyatake K., Nakano Y., Kitaoka S. Purification and some properties of short chain-length specific trans-2-enoyl-CoA reductase in mitochondria of Euglena gracilis. J Biochem. 1986 Oct;100(4):995–1000. doi: 10.1093/oxfordjournals.jbchem.a121813. [DOI] [PubMed] [Google Scholar]
- Ishidate K., Mizugaki M., Uchiyama M. Biohydrogenation accompanying the beta-oxidation of unsaturated fatty acids by Candida. J Biochem. 1973 Aug;74(2):279–283. [PubMed] [Google Scholar]
- Ishidate K., Mizugaki M., Uchiyama M. Induction of NADPH enoyl-coA reductase in Candida grown in the presence of unsaturated fatty acids. J Biochem. 1974 Nov;76(5):1139–1142. [PubMed] [Google Scholar]
- Kawaguchi A., Yoshimura T., Okuda S. A new method for the preparation of acyl-CoA thioesters. J Biochem. 1981 Feb;89(2):337–339. doi: 10.1093/oxfordjournals.jbchem.a133207. [DOI] [PubMed] [Google Scholar]
- Kikuchi S., Kusaka T. Purification of NADPH-dependent enoyl-CoA reductase involved in the malonyl-CoA dependent fatty acid elongation system of Mycobacterium smegmatis. J Biochem. 1984 Sep;96(3):841–848. doi: 10.1093/oxfordjournals.jbchem.a134902. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mizugaki M., Nishimaki T., Shiraishi T., Kawaguchi A., Okuda S., Yamanaka H. Studies on the metabolism of unsaturated fatty acids. IX. Stereochemical studies of the reaction catalyzed by trans-2-enoyl-coenzyme A reductase of Escherichia coli. J Biochem. 1982 Nov;92(5):1649–1654. doi: 10.1093/oxfordjournals.jbchem.a134091. [DOI] [PubMed] [Google Scholar]
- Nishimaki T., Yamanaka H., Mizugaki M. Studies on the metabolism of unsaturated fatty acids. XIV. Purification and properties of NADPH-dependent trans-2-enoyl-CoA reductase of Escherichia coli K-12. J Biochem. 1984 May;95(5):1315–1321. doi: 10.1093/oxfordjournals.jbchem.a134737. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Kawaguchi A., Seyama Y., Yamakawa T., Okuda S. Steric course of deuterium incorporation from [2-2H2]malonyl-CoA into fatty acids by fatty acid synthetases. J Biochem. 1981 Dec;90(6):1697–1704. doi: 10.1093/oxfordjournals.jbchem.a133646. [DOI] [PubMed] [Google Scholar]
- Saito K., Kawaguchi A., Seyama Y., Yamakawa T., Okuda S. Steric course of reaction catalyzed by the enoyl acyl-carrier-protein reductase of Escherichia coli. Eur J Biochem. 1981 Jun 1;116(3):581–586. doi: 10.1111/j.1432-1033.1981.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Kusaka T. Purification and characterization of 2-enoyl-CoA reductase of Mycobacterium smegmatis. J Biochem. 1981 Apr;89(4):1075–1080. [PubMed] [Google Scholar]
- Sugita M., Natori Y., Sasaki T., Furihata K., Shimazu A., Seto H., Otake N. Studies on mycotrienin antibiotics, a novel class of ansamycins. I. Taxonomy, fermentation, isolation and properties of mycotrienins I and II. J Antibiot (Tokyo) 1982 Nov;35(11):1460–1466. doi: 10.7164/antibiotics.35.1460. [DOI] [PubMed] [Google Scholar]
- Wu T. S., Duncan J., Tsao S. W., Chang C. J., Keller P. J., Floss H. G. Biosynthesis of the ansamycin antibiotic ansatrienin (mycotrienin) by Streptomyces collinus. J Nat Prod. 1987 Jan-Feb;50(1):108–118. doi: 10.1021/np50049a015. [DOI] [PubMed] [Google Scholar]