Abstract
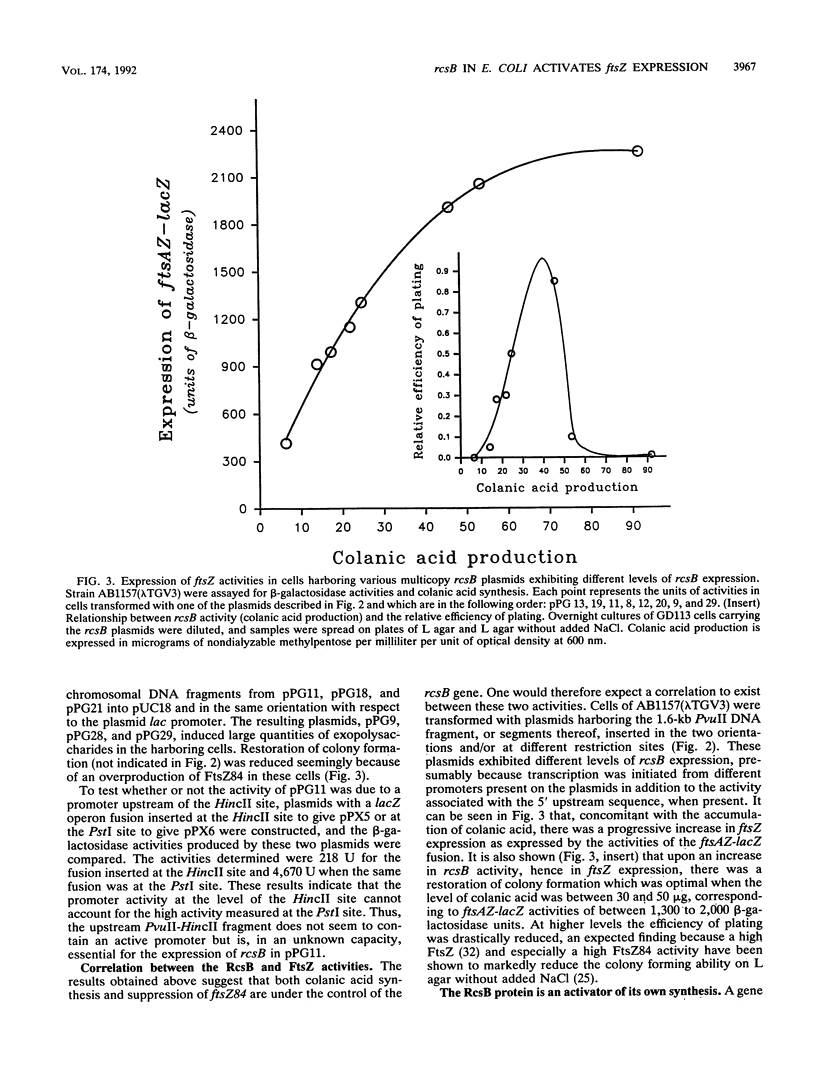
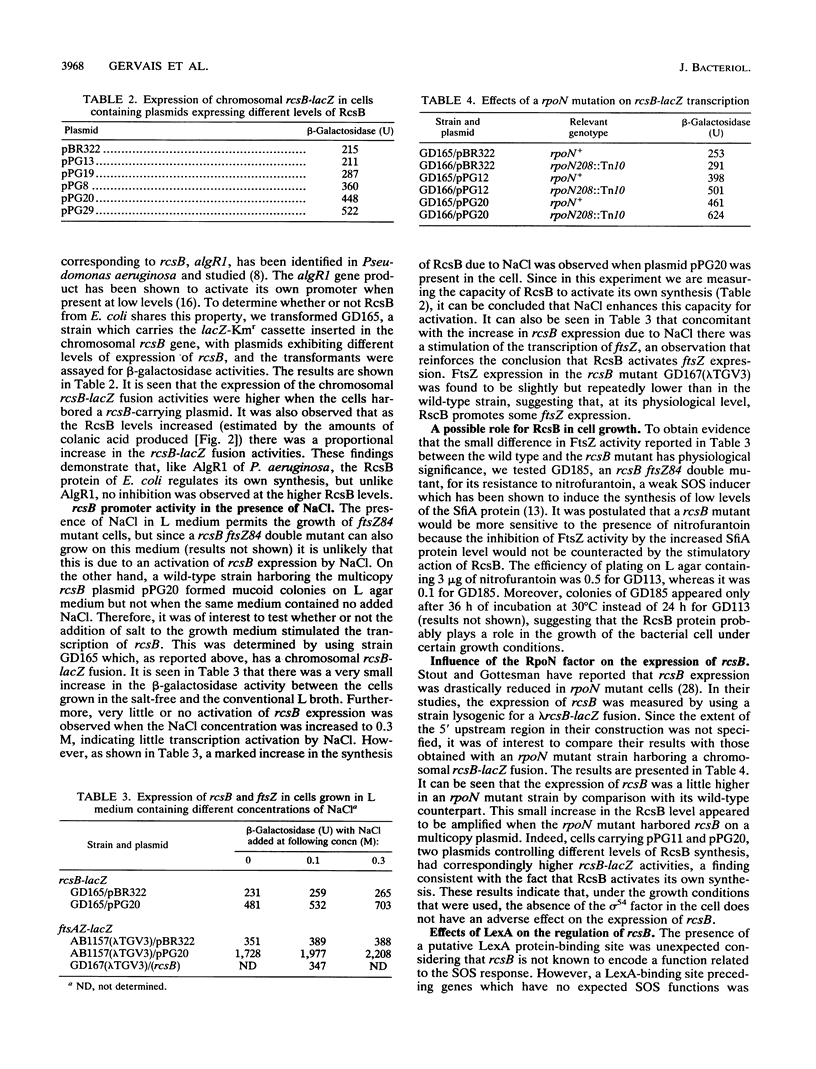
Wild-type genes which, when overexpressed, are capable of restoring the growth deficiency of the division mutant ftsZ84 of Escherichia coli on L medium containing no added NaCl have been isolated. One of these genes is rcsB, a positive regulator of colanic acid biosynthesis. A direct relationship between rcsB expression and FtsZ activity was observed, suggesting that RcsB specifically increases transcription of ftsZ, thus accounting for the restoration of colony formation by ftsZ84 mutant cells. Analysis of the 5' upstream sequence of rcsB revealed, in addition to the sigma 54 promoter sequence previously reported, a presumptive sigma 70 promoter and LexA-binding site plus an upstream sequence that is found to be essential for the expression of rcsB on a plasmid. The absence of the sigma 54 factor does not have a negative effect on the transcription of rcsB. The RcsB protein is an activator of its own synthesis, particularly in the presence of NaCl. Evidence which suggests that RcsB can be phosphorylated by a presumably modified EnvZ or PhoM sensor protein leading to a suppression of the growth deficiency of ftsZ84 mutant cells and to an increase in colanic acid production was obtained. We also demonstrated that the level of colanic acid is reduced when the cells carry a multicopy rcsC plasmid, suggesting that the RcsC sensor has phosphatase activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Garrido T., Pla J., Vicente M. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990 Nov;9(11):3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhumeur P., Drapeau G. R. Regulation of cell division in Escherichia coli: properties of new ftsZ mutants. Mol Gen Genet. 1984;197(2):254–260. doi: 10.1007/BF00330971. [DOI] [PubMed] [Google Scholar]
- Bi E., Lutkenhaus J. FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol. 1990 May;172(5):2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J. A., Quinlan-Walshe C., Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988 Jun;170(6):2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K., Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991 Jun;173(11):3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Mohr C. D., Martin D. W. Mucoid Pseudomonas aeruginosa in cystic fibrosis: signal transduction and histone-like elements in the regulation of bacterial virulence. Mol Microbiol. 1991 Jul;5(7):1577–1583. doi: 10.1111/j.1365-2958.1991.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Deretic V., Tomasek P., Darzins A., Chakrabarty A. M. Gene amplification induces mucoid phenotype in rec-2 Pseudomonas aeruginosa exposed to kanamycin. J Bacteriol. 1986 Feb;165(2):510–516. doi: 10.1128/jb.165.2.510-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991 Jul;5(7):1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Kang S., Markovitz A. Induction of capsular polysaccharide synthesis by rho-fluorophenylalanine in Escherichia coli wild type and strains with altered phenylalanyl soluble ribonucleic acid synthetase. J Bacteriol. 1967 Feb;93(2):584–591. doi: 10.1128/jb.93.2.584-591.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbara K., Chakrabarty A. M. Control of alginate synthesis in Pseudomonas aeruginosa: regulation of the algR1 gene. Biochem Biophys Res Commun. 1989 Oct 31;164(2):601–608. doi: 10.1016/0006-291x(89)91502-7. [DOI] [PubMed] [Google Scholar]
- Kokotek W., Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene. 1989 Dec 14;84(2):467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke D., Bernstein J., Hamilton C., Torriani A. Identification of the phoM gene product and its regulation in Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):19–25. doi: 10.1128/jb.159.1.19-25.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Ruiz A. A., Godson G. N. Promotion, termination, and anti-termination in the rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli K-12. Mol Gen Genet. 1984;195(3):391–401. doi: 10.1007/BF00341439. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- Noël G., Drapeau G. R. Identification of new cell division genes in Escherichia coli by using extragenic suppressors. J Bacteriol. 1986 Feb;165(2):399–404. doi: 10.1128/jb.165.2.399-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix P., Drapeau G. R. Cell division control in Escherichia coli K-12: some properties of the ftsZ84 mutation and suppression of this mutation by the product of a newly identified gene. J Bacteriol. 1988 Sep;170(9):4338–4342. doi: 10.1128/jb.170.9.4338-4342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno-Nishio S., Backman K. C., Magasanik B. Regulation at the glnL-operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1247–1251. doi: 10.1128/jb.153.3.1247-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985 Oct;42(3):941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q. M., Rockenbach S., Ward J. E., Jr, Lutkenhaus J. Structure and expression of the cell division genes ftsQ, ftsA and ftsZ. J Mol Biol. 1985 Aug 5;184(3):399–412. doi: 10.1016/0022-2836(85)90290-6. [DOI] [PubMed] [Google Scholar]



