Abstract
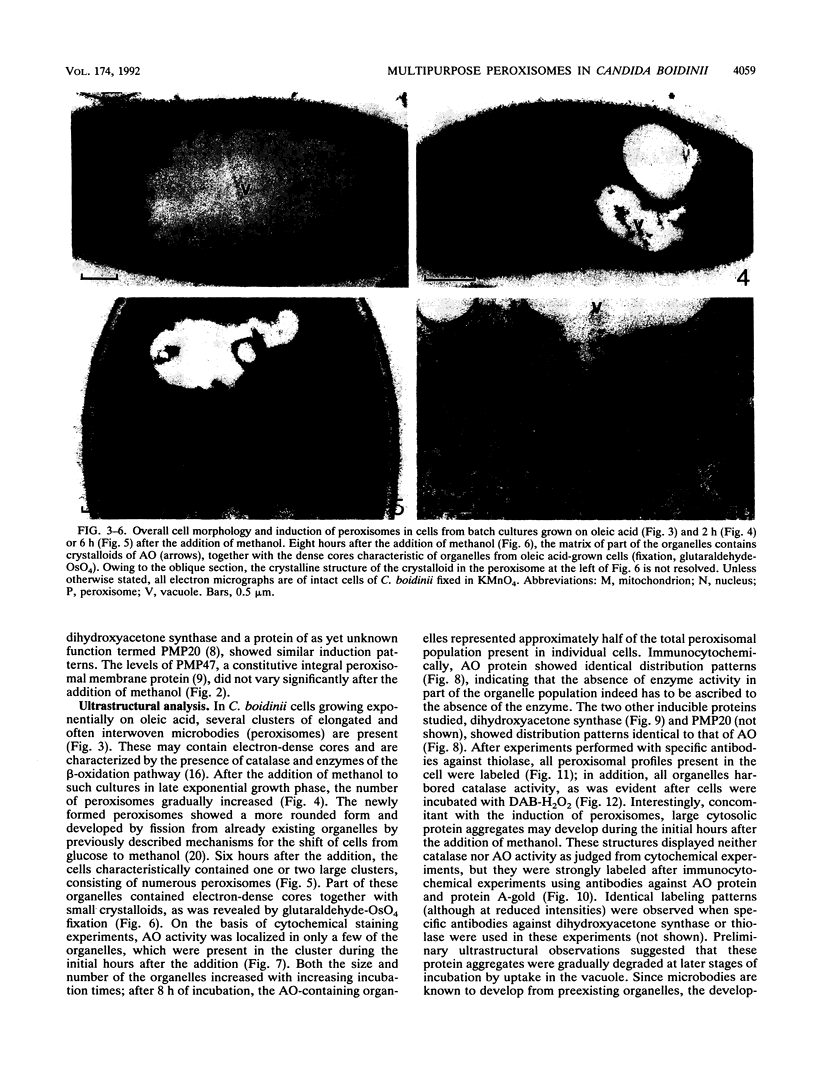
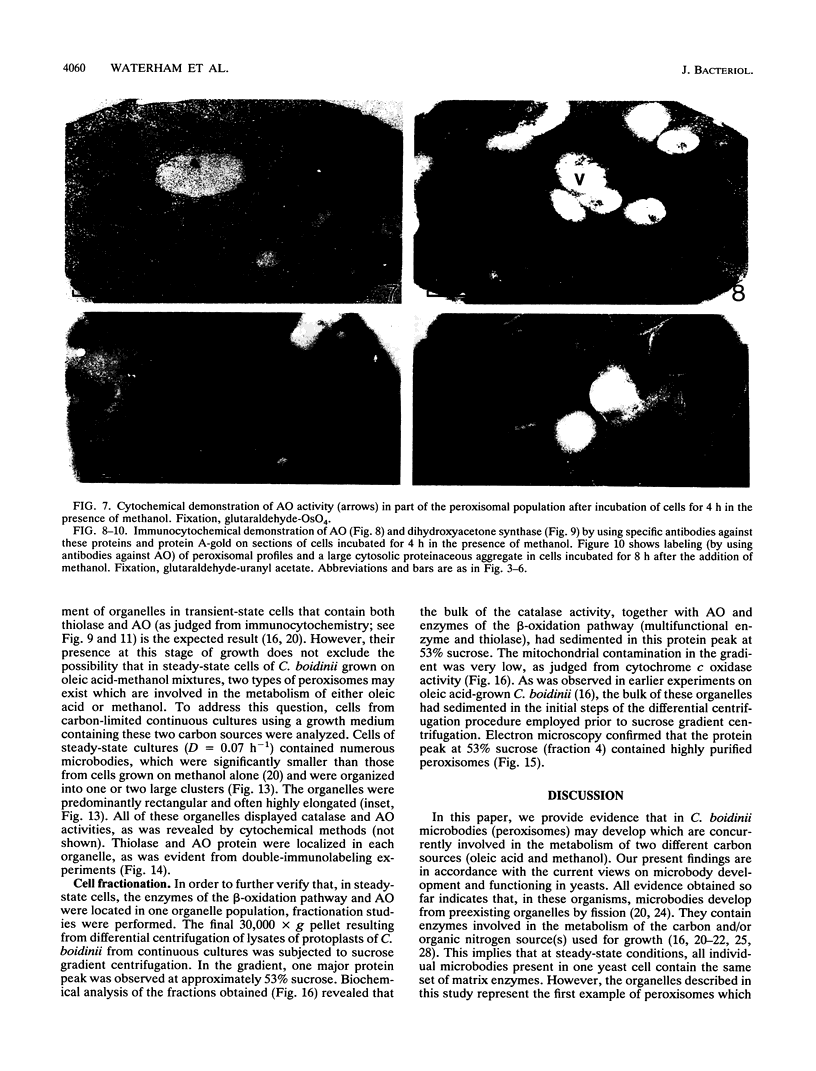
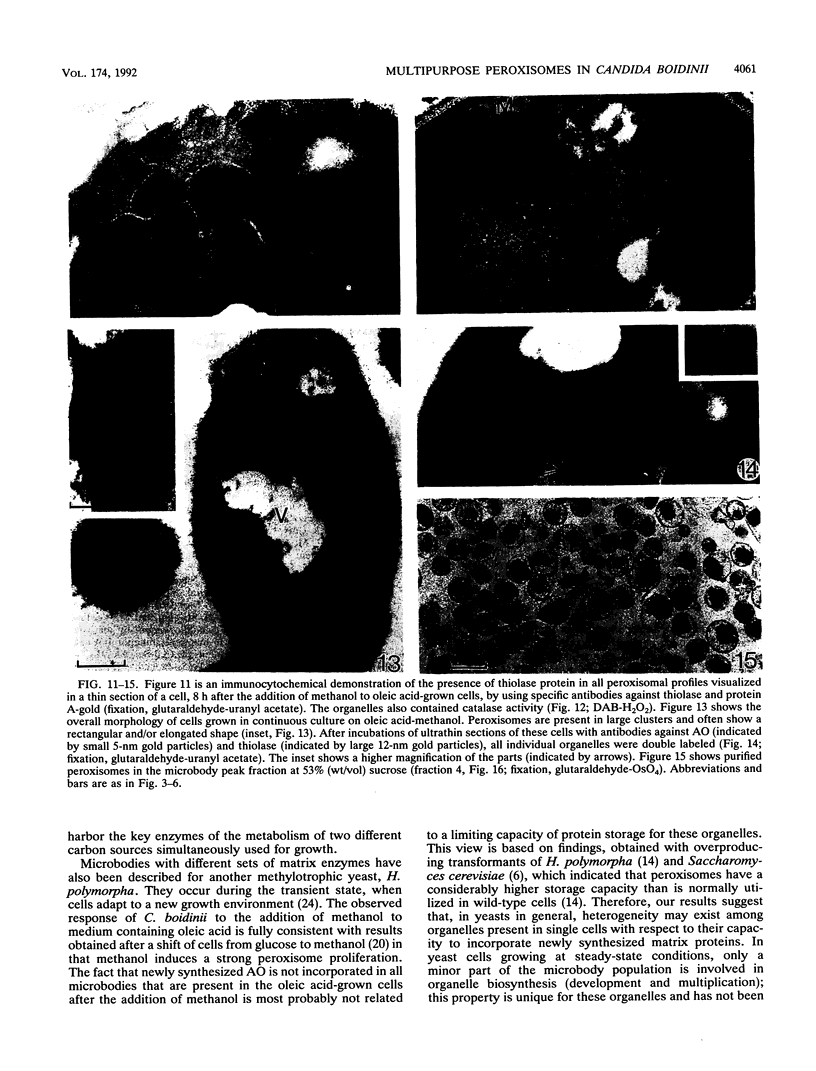
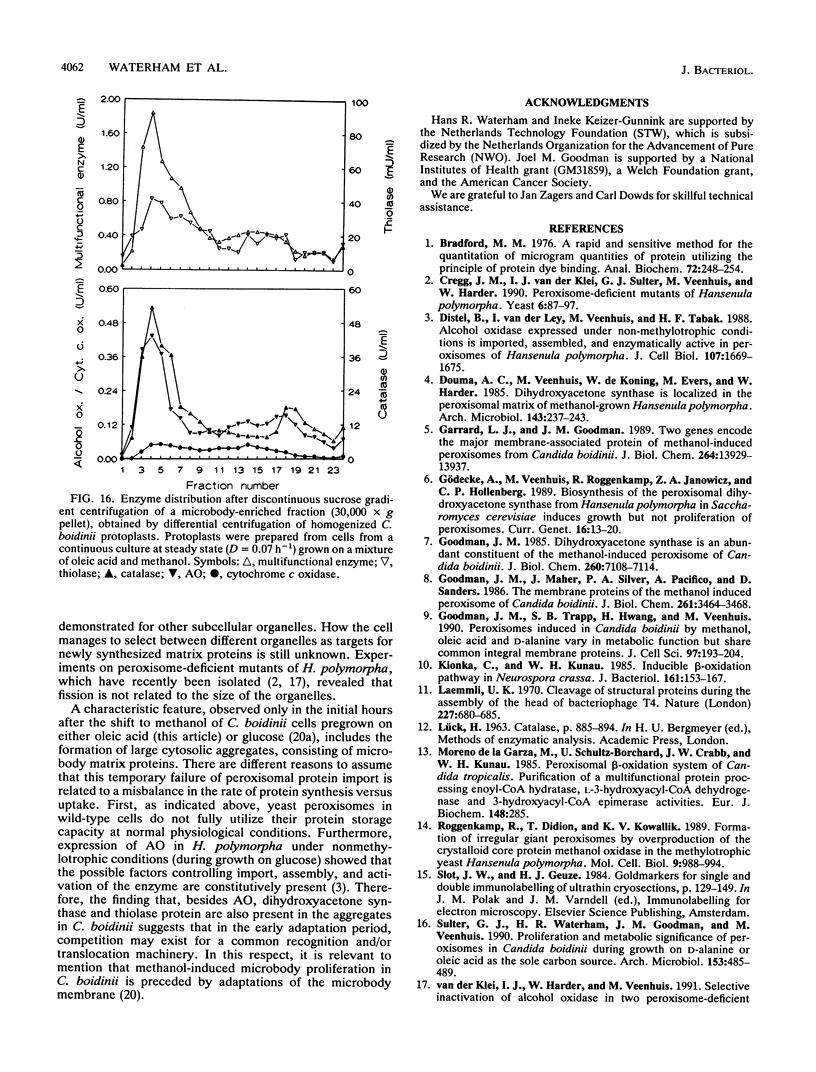
We have studied the development and metabolic significance of peroxisomes in the yeast Candida boidinii following adaptation of the organism to cultivation conditions which require the simultaneous presence and activity of two independent peroxisome-mediated pathways for growth. After the addition of methanol to oleic acid-grown cells at late exponentional growth, a number of new small peroxisomes developed which, apart from the presence of beta-oxidation enzymes, were characterized by the presence of enzymes involved in methanol metabolism (alcohol oxidase and dihydroxyacetone synthase). The latter proteins, however, were absent in the larger organelles which were originally present in the oleic acid-grown cells prior to the addition of methanol and which contained only enzymes of the beta-oxidation pathway. Subsequent experiments on cells from continuous cultures grown on a mixture of oleic acid and methanol at steady-state conditions revealed that both the enzymes of the beta-oxidation pathway and those involved in methanol metabolism were found in one and the same compartment. Thus, under these conditions the cells contained peroxisomes which were concurrently involved in the metabolism of two different carbon sources simultaneously used for growth. Our results indicated that the heterogeneity in the peroxisomal population of a single cell, observed in the transient state following the addition of methanol, is only temporary and due to heterogeneity among these organelles with respect to their capacity to incorporate newly synthesized matrix proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Distel B., Van der Leÿ I., Veenhuis M., Tabak H. F. Alcohol oxidase expressed under nonmethylotrophic conditions is imported, assembled, and enzymatically active in peroxisomes of Hansenula polymorpha. J Cell Biol. 1988 Nov;107(5):1669–1675. doi: 10.1083/jcb.107.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard L. J., Goodman J. M. Two genes encode the major membrane-associated protein of methanol-induced peroxisomes from Candida boidinii. J Biol Chem. 1989 Aug 15;264(23):13929–13937. [PubMed] [Google Scholar]
- Goodman J. M. Dihydroxyacetone synthase is an abundant constituent of the methanol-induced peroxisome of Candida boidinii. J Biol Chem. 1985 Jun 10;260(11):7108–7113. [PubMed] [Google Scholar]
- Goodman J. M., Maher J., Silver P. A., Pacifico A., Sanders D. The membrane proteins of the methanol-induced peroxisome of Candida boidinii. Initial characterization and generation of monoclonal antibodies. J Biol Chem. 1986 Mar 5;261(7):3464–3468. [PubMed] [Google Scholar]
- Goodman J. M., Trapp S. B., Hwang H., Veenhuis M. Peroxisomes induced in Candida boidinii by methanol, oleic acid and D-alanine vary in metabolic function but share common integral membrane proteins. J Cell Sci. 1990 Sep;97(Pt 1):193–204. doi: 10.1242/jcs.97.1.193. [DOI] [PubMed] [Google Scholar]
- Gödecke A., Veenhuis M., Roggenkamp R., Janowicz Z. A., Hollenberg C. P. Biosynthesis of the peroxisomal dihydroxyacetone synthase from Hansenula polymorpha in Saccharomyces cerevisiae induces growth but not proliferation of peroxisomes. Curr Genet. 1989 Jul;16(1):13–20. doi: 10.1007/BF00411078. [DOI] [PubMed] [Google Scholar]
- Kionka C., Kunau W. H. Inducible beta-oxidation pathway in Neurospora crassa. J Bacteriol. 1985 Jan;161(1):153–157. doi: 10.1128/jb.161.1.153-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moreno de la Garza M., Schultz-Borchard U., Crabb J. W., Kunau W. H. Peroxisomal beta-oxidation system of Candida tropicalis. Purification of a multifunctional protein possessing enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase and 3-hydroxyacyl-CoA epimerase activities. Eur J Biochem. 1985 Apr 15;148(2):285–291. doi: 10.1111/j.1432-1033.1985.tb08837.x. [DOI] [PubMed] [Google Scholar]
- Roggenkamp R., Didion T., Kowallik K. V. Formation of irregular giant peroxisomes by overproduction of the crystalloid core protein methanol oxidase in the methylotrophic yeast Hansenula polymorpha. Mol Cell Biol. 1989 Mar;9(3):988–994. doi: 10.1128/mcb.9.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulter G. J., Waterham H. R., Goodman J. M., Veenhuis M. Proliferation and metabolic significance of peroxisomes in Candida boidinii during growth on D-alanine or oleic acid as the sole carbon source. Arch Microbiol. 1990;153(5):485–489. doi: 10.1007/BF00248431. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Goodman J. M. Peroxisomal assembly: membrane proliferation precedes the induction of the abundant matrix proteins in the methylotrophic yeast Candida boidinii. J Cell Sci. 1990 Aug;96(Pt 4):583–590. doi: 10.1242/jcs.96.4.583. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Harder W. Microbodies in yeasts: structure, function and biogenesis. Microbiol Sci. 1988 Nov;5(11):347–351. [PubMed] [Google Scholar]
- Veenhuis M., Sulter G., van der Klei I., Harder W. Evidence for functional heterogeneity among microbodies in yeasts. Arch Microbiol. 1989;151(2):105–110. doi: 10.1007/BF00414422. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Vermeulen C. A., Harder W. Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Nov 7;105(3):261–267. doi: 10.1007/BF00447145. [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Harder W., Veenhuis M. Selective inactivation of alcohol oxidase in two peroxisome-deficient mutants of the yeast Hansenula polymorpha. Yeast. 1991 Nov;7(8):813–821. doi: 10.1002/yea.320070806. [DOI] [PubMed] [Google Scholar]