Abstract
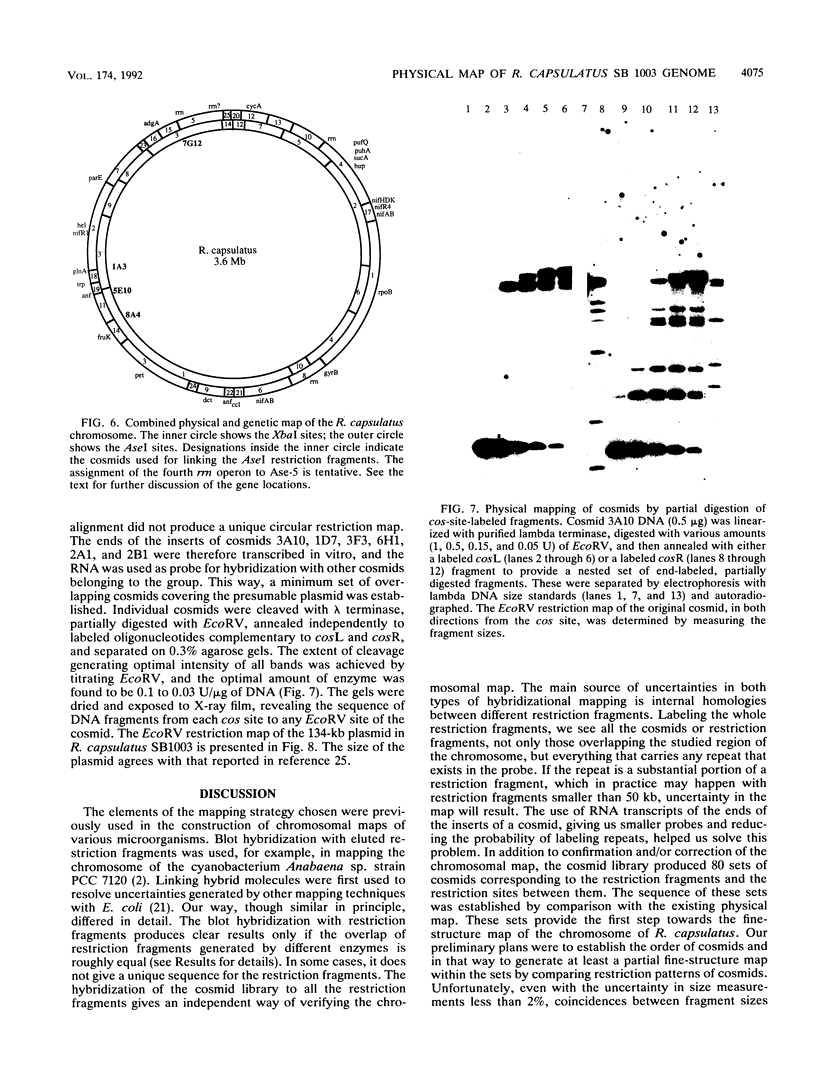
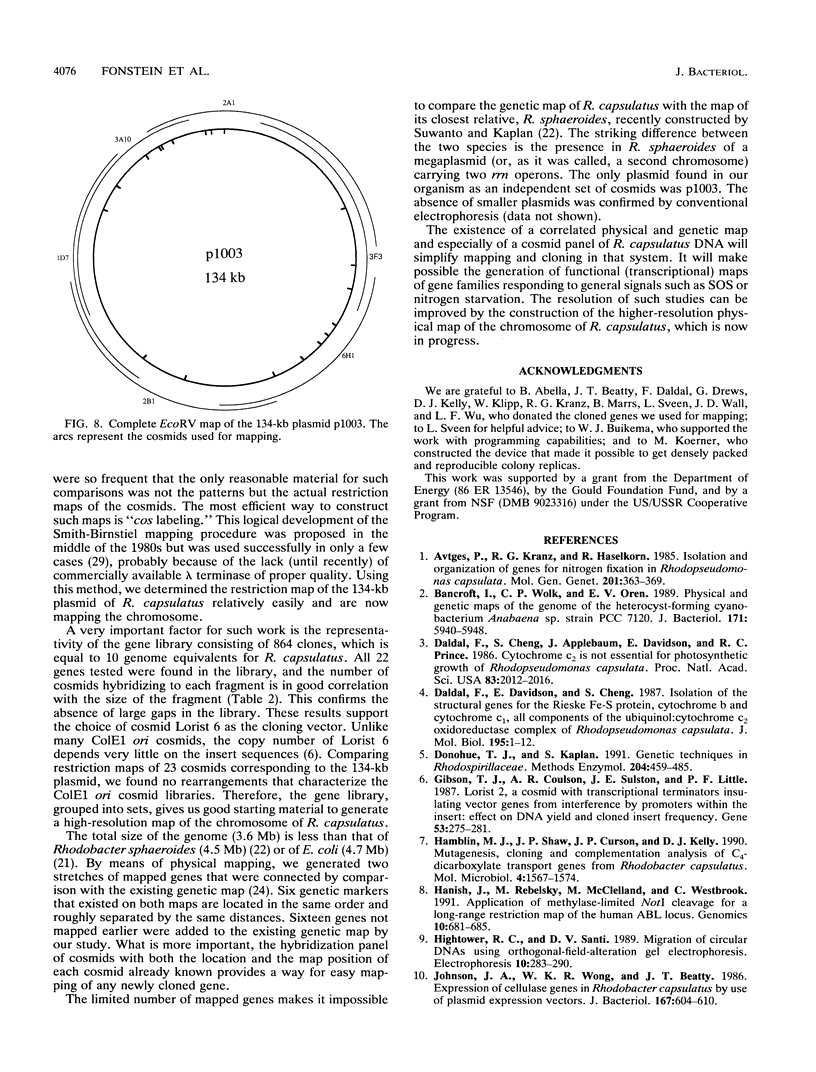
A map of the chromosome of Rhodobacter capsulatus was constructed by overlapping the large restriction fragments generated by endonucleases AseI and XbaI. The analyses were done by hybridization of single fragments with the restriction fragments blotted from pulsed-field gels and by grouping cosmids of a genomic library of R. capsulatus into contigs, corresponding to the restriction fragments, and further overlapping of the contigs. A technical difficulty due to a repeated sequence made it necessary to use hybridization with cloned genes and prior knowledge of the genetic map in order to close the physical circle in a unique way. In all, 41 restriction sites were mapped on the 3.6-Mb circular genome and 22 genes were positioned at 26 loci of the map. Cosmid clones were grouped in about 80 subcontigs, forming two groups, one corresponding to the chromosome of R. capsulatus and the other corresponding to a 134-kb plasmid. cos site end labeling and partial digestion of cosmids were used to construct a high-resolution EcoRV map of the 134-kb plasmid. The same method can be extended to the entire chromosome. The cosmid clones derived in this work can be used as a hybridization panel for the physical mapping of new genes as soon as they are cloned.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avtges P., Kranz R. G., Haselkorn R. Isolation and organization of genes for nitrogen fixation in Rhodopseudomonas capsulata. Mol Gen Genet. 1985;201(3):363–369. doi: 10.1007/BF00331324. [DOI] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Davidson E., Cheng S. Isolation of the structural genes for the Rieske Fe-S protein, cytochrome b and cytochrome c1 all components of the ubiquinol: cytochrome c2 oxidoreductase complex of Rhodopseudomonas capsulata. J Mol Biol. 1987 May 5;195(1):1–12. doi: 10.1016/0022-2836(87)90322-6. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Kaplan S. Genetic techniques in rhodospirillaceae. Methods Enzymol. 1991;204:459–485. doi: 10.1016/0076-6879(91)04024-i. [DOI] [PubMed] [Google Scholar]
- Gibson T. J., Coulson A. R., Sulston J. E., Little P. F. Lorist2, a cosmid with transcriptional terminators insulating vector genes from interference by promoters within the insert: effect on DNA yield and cloned insert frequency. Gene. 1987;53(2-3):275–281. doi: 10.1016/0378-1119(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Hamblin M. J., Shaw J. G., Curson J. P., Kelly D. J. Mutagenesis, cloning and complementation analysis of C4-dicarboxylate transport genes from Rhodobacter capsulatus. Mol Microbiol. 1990 Sep;4(9):1567–1574. [PubMed] [Google Scholar]
- Hanish J., Rebelsky M., McClelland M., Westbrook C. Application of methylase-limited partial NotI cleavage for a long-range restriction map of the human ABL locus. Genomics. 1991 Jul;10(3):681–685. doi: 10.1016/0888-7543(91)90452-k. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Santi D. V. Migration properties of circular DNAs using orthogonal-field-alternation gel electrophoresis. Electrophoresis. 1989 May-Jun;10(5-6):283–290. doi: 10.1002/elps.1150100503. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Wong W. K., Beatty J. T. Expression of cellulase genes in Rhodobacter capsulatus by use of plasmid expression vectors. J Bacteriol. 1986 Aug;167(2):604–610. doi: 10.1128/jb.167.2.604-610.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipp W., Masepohl B., Pühler A. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol. 1988 Feb;170(2):693–699. doi: 10.1128/jb.170.2.693-699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Komine Y., Inokuchi H. Precise mapping of the rnpB gene encoding the RNA component of RNase P in Escherichia coli K-12. J Bacteriol. 1991 Mar;173(5):1813–1816. doi: 10.1128/jb.173.5.1813-1816.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Characterization of nif regulatory genes in Rhodopseudomonas capsulata using lac gene fusions. Gene. 1985;40(2-3):203–215. doi: 10.1016/0378-1119(85)90043-5. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Saffran W., Welsh J., Haas R., Goldenberg M., Cantor C. R. New techniques for purifying large DNAs and studying their properties and packaging. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):189–195. doi: 10.1101/sqb.1983.047.01.024. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Virosco J., Haselkorn R. The wild-type gene for glutamine synthetase restores ammonia control of nitrogen fixation to Gln- (glnA) mutants of Rhodopseudomonas capsulata. J Bacteriol. 1983 Jul;155(1):180–185. doi: 10.1128/jb.155.1.180-185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Braddock K. Mapping of Rhodopseudomonas capsulata nif genes. J Bacteriol. 1984 May;158(2):404–410. doi: 10.1128/jb.158.2.404-410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. W., Wall J. D. Clustering of genes necessary for hydrogen oxidation in Rhodobacter capsulatus. J Bacteriol. 1991 Apr;173(7):2401–2405. doi: 10.1128/jb.173.7.2401-2405.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Zuber U., Schumann W. Tn5cos: a transposon for restriction mapping of large plasmids using phage lambda terminase. Gene. 1991 Jul 15;103(1):69–72. doi: 10.1016/0378-1119(91)90392-o. [DOI] [PubMed] [Google Scholar]