Abstract
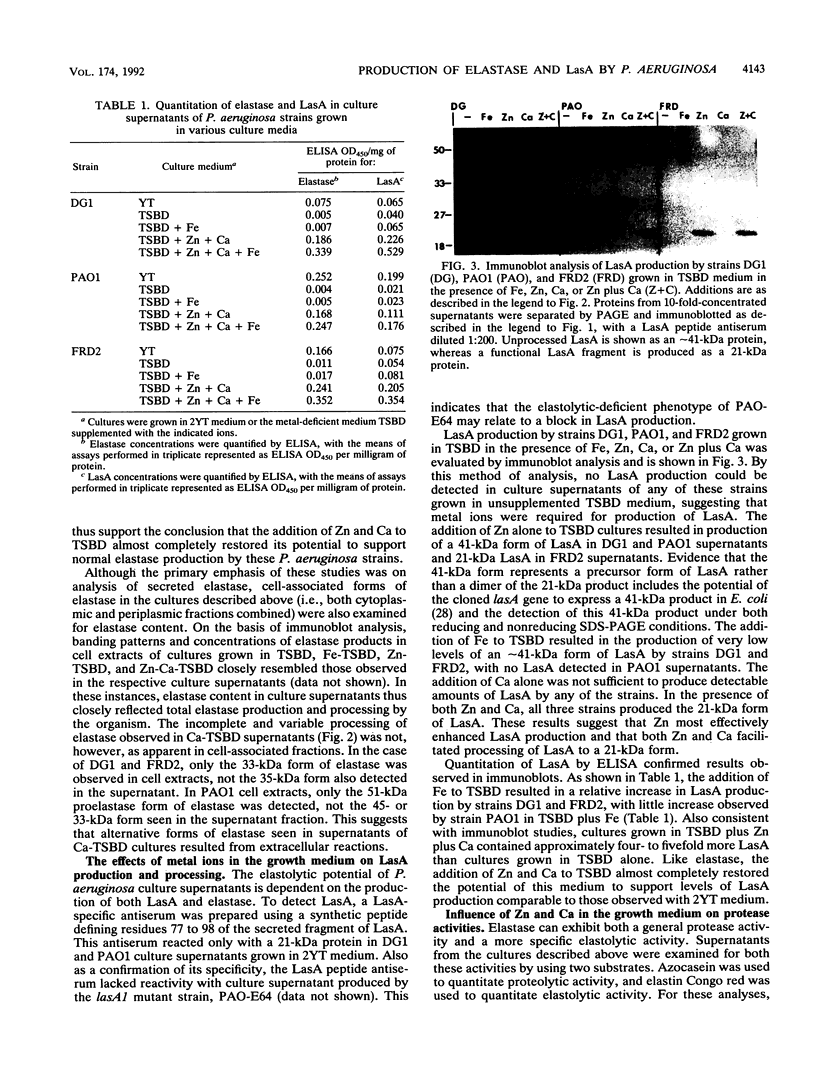
The ability of Pseudomonas aeruginosa to degrade elastin, a major component of connective tissue, likely contributes to its pathogenicity and multiplication in human tissues. Two extracellular enzymes are required for P. aeruginosa elastolytic activity: elastase and LasA. Elastase is a zinc metalloprotease, but little is known about the structure of LasA. When grown under metal ion-deficient conditions, P. aeruginosa culture supernatants were found to exhibit a low level of elastolytic activity, which coincided with production of low levels of the 51-kDa proelastase and no detectable LasA. By using this fact to identify factors that promote elastolytic activity, P. aeruginosa PAO1, FRD2, and DG1 were grown in metal ion-deficient medium supplemented with zinc (10(-4) M ZnCl2), calcium (2.5 x 10(-3) M CaCl2), or iron (10(-4) M FeCl3). High levels of proteolytic and elastolytic activity were exhibited by all strains when cultured in the presence of both zinc and calcium, and this was associated with the production of mature 33-kDa elastase and 21-kDa LasA. Supplementing DG1 and PAO1 cultures with zinc alone stimulated the production of 33-kDa elastase, which, because of the calcium-deficient conditions, exhibited low proteolytic and elastolytic activities. Zinc also stimulated the production of a 41-kDa form of LasA in DG1 and PAO1 culture supernatants. Elastase production by FRD2 cultured in the presence of zinc alone differed from that by the other two strains in that supernatants contained 33-kDa elastase, a 21-kDa form of LasA, and exhibited high proteolytic and elastolytic activities. Such strain-associated differences in LasA processing and elastase activity can be explained by differences in metal ion-scavenging mechanisms adapted by the strains. Supplementing cultures with calcium stimulated the production of elastase but had no effect on LasA production. The elastase produced exhibited variable sizes, possibly resulting from aberrant processing reactions, and showed little proteolytic activity. Proteolytic activity could be recovered from 33-kDa elastase produced in the presence of calcium by inclusion of zinc in the enzymatic assay. Although iron was previously found to exert a repressive effect on P. aeruginosa elastolytic activity, iron exerted little effect on elastolytic activity when added to cultures containing both zinc and calcium. These studies support the conclusion that elastase production and processing are promoted by both zinc and calcium. LasA production, in comparison, is stimulated by zinc, with both zinc and calcium facilitating its processing. The association of 41-kDa LasA with a low level of elastolytic activity and of 21-kDa LasA with a high level of activity supports the conclusion that lasA encodes a larger, precursor protein which is processed to an active 21-kDa form during secretion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M. J., Storey D. G. Zinc and iron regulate translation of the gene encoding Pseudomonas aeruginosa elastase. Mol Microbiol. 1992 Feb;6(3):337–344. doi: 10.1111/j.1365-2958.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Darzins A., Peters J. E., Galloway D. R. Revised nucleotide sequence of the lasA gene from Pseudomonas aeruginosa PAO1. Nucleic Acids Res. 1990 Nov 11;18(21):6444–6444. doi: 10.1093/nar/18.21.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. II. Einwirkung zweier gereinigter Proteasen auf die menschlichen Immunoglobuline IgG, IgA und sekretorisches IgA. Zentralbl Bakteriol A. 1981 Mar;249(1):89–98. [PubMed] [Google Scholar]
- Fukushima J., Yamamoto S., Morihara K., Atsumi Y., Takeuchi H., Kawamoto S., Okuda K. Structural gene and complete amino acid sequence of Pseudomonas aeruginosa IFO 3455 elastase. J Bacteriol. 1989 Mar;171(3):1698–1704. doi: 10.1128/jb.171.3.1698-1704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Activation of an elastase precursor by the lasA gene product of Pseudomonas aeruginosa. J Bacteriol. 1987 Oct;169(10):4532–4539. doi: 10.1128/jb.169.10.4532-4539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and transcriptional regulation of the elastase lasA gene in mucoid and nonmucoid Pseudomonas aeruginosa. J Bacteriol. 1987 Mar;169(3):1349–1351. doi: 10.1128/jb.169.3.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Alarcon P. G., Kulhavy R. M., Morihara K., Russell M. W., Mestecky J. F. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol. 1990 Mar 15;144(6):2253–2257. [PubMed] [Google Scholar]
- Heck L. W., Morihara K., McRae W. B., Miller E. J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986 Jan;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- Holloway B. W., Morgan A. F. Genome organization in Pseudomonas. Annu Rev Microbiol. 1986;40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- Kessler E., Israel M., Landshman N., Chechick A., Blumberg S. In vitro inhibition of Pseudomonas aeruginosa elastase by metal-chelating peptide derivatives. Infect Immun. 1982 Nov;38(2):716–723. doi: 10.1128/iai.38.2.716-723.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E., Safrin M. Synthesis, processing, and transport of Pseudomonas aeruginosa elastase. J Bacteriol. 1988 Nov;170(11):5241–5247. doi: 10.1128/jb.170.11.5241-5247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- McIver K., Kessler E., Ohman D. E. Substitution of active-site His-223 in Pseudomonas aeruginosa elastase and expression of the mutated lasB alleles in Escherichia coli show evidence for autoproteolytic processing of proelastase. J Bacteriol. 1991 Dec;173(24):7781–7789. doi: 10.1128/jb.173.24.7781-7789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Cryz S. J., Iglewski B. H. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980 Jun;142(3):836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. C., Hamood A. N., Vincent T. S., Beachey E. H., Iglewski B. H. Identification of functional epitopes of Pseudomonas aeruginosa exotoxin A using synthetic peptides and subclone products. Mol Immunol. 1990 Oct;27(10):981–993. doi: 10.1016/0161-5890(90)90121-f. [DOI] [PubMed] [Google Scholar]
- Peters J. E., Galloway D. R. Purification and characterization of an active fragment of the LasA protein from Pseudomonas aeruginosa: enhancement of elastase activity. J Bacteriol. 1990 May;172(5):2236–2240. doi: 10.1128/jb.172.5.2236-2240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad P. A., Iglewski B. H. Nucleotide sequence and expression in Escherichia coli of the Pseudomonas aeruginosa lasA gene. J Bacteriol. 1988 Jun;170(6):2784–2789. doi: 10.1128/jb.170.6.2784-2789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Kang A. H., Beachey E. H. Primary structural similarities between types 5 and 24 M proteins of Streptococcus pyogenes. Biochem Biophys Res Commun. 1980 Jan 29;92(2):546–553. doi: 10.1016/0006-291x(80)90368-x. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Kawamoto S., Fukushima J., Hamajima K., Onishi H., Miyagi Y., Inami S., Morihara K., Okuda K. Detection of elastase production in Escherichia coli with the elastase structural gene from several non-elastase-producing strains of Pseudomonas aeruginosa. J Bacteriol. 1991 Oct;173(19):6153–6158. doi: 10.1128/jb.173.19.6153-6158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. M., Flaherty K. M., McKay D. B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J Biol Chem. 1991 Feb 15;266(5):2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Que J. U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987 Mar;55(3):579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Hedén L., Sjöberg L., Wadström T. Production of enzymes and toxins by hospital strains of Pseudomonas aeruginosa in relation to serotype and phage-typing pattern. J Med Microbiol. 1973 Feb;6(1):91–100. doi: 10.1099/00222615-6-1-91. [DOI] [PubMed] [Google Scholar]







