Abstract
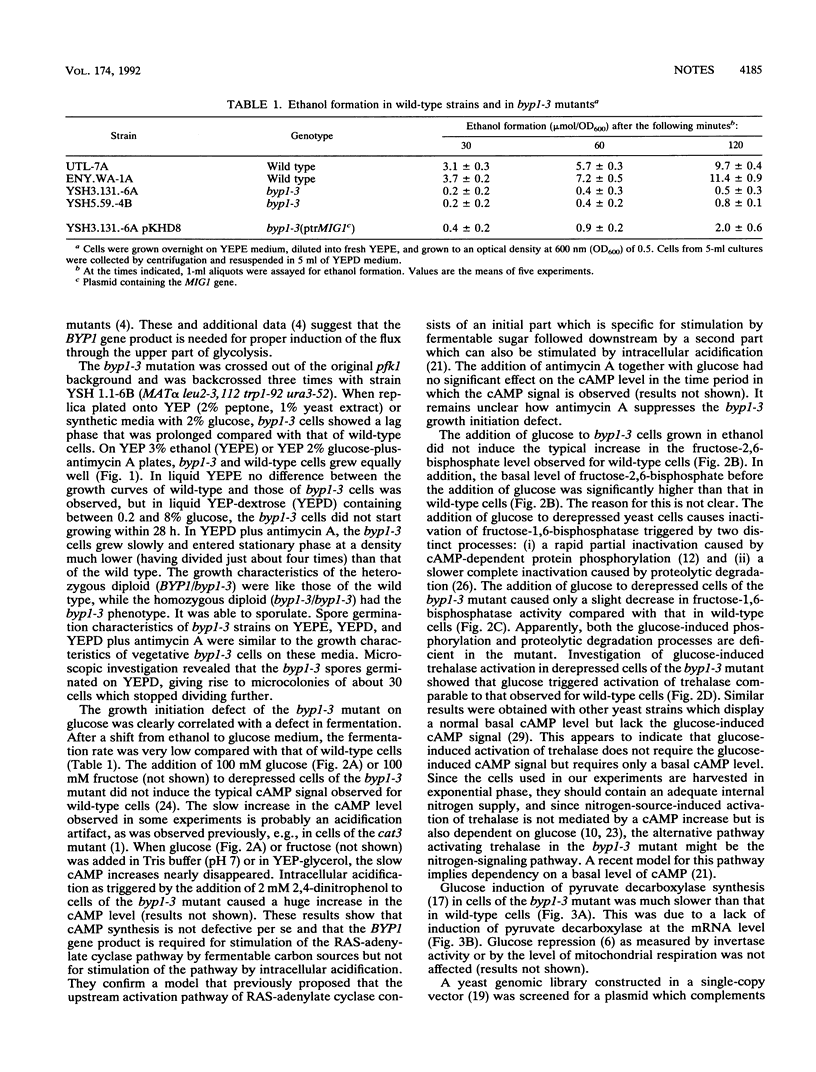
Saccharomyces cerevisiae byp1-3 mutants displayed a long lag phase when shifted from a nonfermentable carbon source to a medium containing glucose. The byp1-3 mutation also caused several defects in regulatory phenomena which occur during the transition from the derepressed state to the repressed state. As opposed to wild-type cells, the addition of glucose to cells of the byp1-3 mutant grown on nonfermentable carbon sources did not induce a cyclic AMP signal. Fructose-2,6-bisphosphate formation and inactivation of fructose-1,6-bisphosphatase were severely delayed, but trehalase activation was not affected. In addition, the induction of pyruvate decarboxylase both at the level of activity and that of transcription was very slow compared with that in wild-type cells. These pleotropic defects in glucose-induced regulatory phenomena might be responsible for the very long lag phase of byp1-3 cells and the inability of ascospores to initiate growth after germination on glucose media. Screening of a yeast gene library for clones complementing the byp1-3 phenotype resulted in the isolation of a truncated form of the previously described zinc finger transcription repressor MIG1. The entire MIG1 gene and the truncated form suppressed even on a single-copy vector the growth initiation defect but not the regulatory abnormalities of the byp1-3 mutant. MIG1 is not allelic to byp1-3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argüelles J. C., Mbonyi K., Van Aelst L., Vanhalewyn M., Jans A. W., Thevelein J. M. Absence of glucose-induced cAMP signaling in the Saccharomyces cerevisiae mutants cat1 and cat3 which are deficient in derepression of glucose-repressible proteins. Arch Microbiol. 1990;154(2):199–205. doi: 10.1007/BF00423333. [DOI] [PubMed] [Google Scholar]
- Beullens M., Mbonyi K., Geerts L., Gladines D., Detremerie K., Jans A. W., Thevelein J. M. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1988 Feb 15;172(1):227–231. doi: 10.1111/j.1432-1033.1988.tb13877.x. [DOI] [PubMed] [Google Scholar]
- Charlab R., Oliveira D. E., Panek A. D. Investigation of the relationship between sst1 and fdp mutations in yeast and their effect on trehalose synthesis. Braz J Med Biol Res. 1985;18(4):447–454. [PubMed] [Google Scholar]
- Entian K. D. Glucose repression: a complex regulatory system in yeast. Microbiol Sci. 1986 Dec;3(12):366–371. [PubMed] [Google Scholar]
- François J., Van Schaftingen E., Hers H. G. The mechanism by which glucose increases fructose 2,6-bisphosphate concentration in Saccharomyces cerevisiae. A cyclic-AMP-dependent activation of phosphofructokinase 2. Eur J Biochem. 1984 Nov 15;145(1):187–193. doi: 10.1111/j.1432-1033.1984.tb08539.x. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonyi K., Beullens M., Detremerie K., Geerts L., Thevelein J. M. Requirement of one functional RAS gene and inability of an oncogenic ras variant to mediate the glucose-induced cyclic AMP signal in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1988 Aug;8(8):3051–3057. doi: 10.1128/mcb.8.8.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonyi K., van Aelst L., Argüelles J. C., Jans A. W., Thevelein J. M. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1990 Sep;10(9):4518–4523. doi: 10.1128/mcb.10.9.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H. D., Ciriacy M., Zimmermann F. K. The synthesis of yeast pyruvate decarboxylase is regulated by large variations in the messenger RNA level. Mol Gen Genet. 1983;192(1-2):247–252. doi: 10.1007/BF00327674. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Zimmermann F. K. Genetic analysis of the pyruvate decarboxylase reaction in yeast glycolysis. J Bacteriol. 1982 Sep;151(3):1146–1152. doi: 10.1128/jb.151.3.1146-1152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengstag C., Hinnen A. The sequence of the Saccharomyces cerevisiae gene PHO2 codes for a regulatory protein with unusual aminoacid composition. Nucleic Acids Res. 1987 Jan 12;15(1):233–246. doi: 10.1093/nar/15.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M. Cyclic AMP and the stimulation of trehalase activity in the yeast Saccharomyces cerevisiae by carbon sources, nitrogen sources and inhibitors of protein synthesis. J Gen Microbiol. 1985 Dec;131(12):3199–3209. doi: 10.1099/00221287-131-12-3199. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M., Beullens M., Honshoven F., Hoebeeck G., Detremerie K., den Hollander J. A., Jans A. W. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987 Aug;133(8):2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M. Fermentable sugars and intracellular acidification as specific activators of the RAS-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol Microbiol. 1991 Jun;5(6):1301–1307. doi: 10.1111/j.1365-2958.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Tortora P., Birtel M., Lenz A. G., Holzer H. Glucose-dependent metabolic interconversion of fructose-1, 6-bisphosphatase in yeast. Biochem Biophys Res Commun. 1981 May 29;100(2):688–695. doi: 10.1016/s0006-291x(81)80230-6. [DOI] [PubMed] [Google Scholar]
- Tortora P., Burlini N., Caspani G., Guerritore A. Studies on glucose-induced inactivation of gluconeogenetic enzymes in adenylate cyclase and cAMP-dependent protein kinase yeast mutants. Eur J Biochem. 1984 Dec 17;145(3):543–548. doi: 10.1111/j.1432-1033.1984.tb08590.x. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Boy-Marcotte E., Camonis J. H., Thevelein J. M., Jacquet M. The C-terminal part of the CDC25 gene product plays a key role in signal transduction in the glucose-induced modulation of cAMP level in Saccharomyces cerevisiae. Eur J Biochem. 1990 Nov 13;193(3):675–680. doi: 10.1111/j.1432-1033.1990.tb19386.x. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Hohmann S., Zimmermann F. K., Jans A. W., Thevelein J. M. A yeast homologue of the bovine lens fibre MIP gene family complements the growth defect of a Saccharomyces cerevisiae mutant on fermentable sugars but not its defect in glucose-induced RAS-mediated cAMP signalling. EMBO J. 1991 Aug;10(8):2095–2104. doi: 10.1002/j.1460-2075.1991.tb07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- van Aelst L., Jans A. W., Thevelein J. M. Involvement of the CDC25 gene product in the signal transmission pathway of the glucose-induced RAS-mediated cAMP signal in the yeast Saccharomyces cerevisiae. J Gen Microbiol. 1991 Feb;137(2):341–349. doi: 10.1099/00221287-137-2-341. [DOI] [PubMed] [Google Scholar]