Abstract
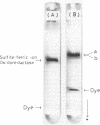
Sulfite:ferric ion oxidoreductase in the plasma membrane of Thiobacillus ferrooxidans AP19-3 was purified to an electrophoretically homogeneous state. The enzyme had an apparent molecular weight of 650,000 and was composed of two subunits (M(rs), 61,000 and 59,000) as estimated by sodium sulfate-polyacrylamide gel electrophoresis. The Michaelis constants of sulfite:ferric ion oxidoreductase for Fe3+ and sulfite ions were 1.0 and 0.071 mM, respectively. Sulfite:ferric ion oxidoreductase suffered from end product inhibition by 1 mM Fe2+.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair F. W. Membrane-associated sulfur oxidation by the autotroph Thiobacillus thiooxidans. J Bacteriol. 1966 Oct;92(4):899–904. doi: 10.1128/jb.92.4.899-904.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONDON J., RITTENBERG S. C. PATH OF SULFUR IN SULFIDE AND THIOSULFATE OXIDATION BY THIOBACILLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1183–1190. doi: 10.1073/pnas.52.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: cytochrome c oxidoreductase. Can J Biochem. 1970 Mar;48(3):334–343. doi: 10.1139/o70-056. [DOI] [PubMed] [Google Scholar]
- Moriarty D. J., Nicholas D. J. Electron transfer during sulphide and sulphite oxidation by Thiobacillus concretivorus. Biochim Biophys Acta. 1970 Aug 4;216(1):130–138. doi: 10.1016/0005-2728(70)90165-9. [DOI] [PubMed] [Google Scholar]
- Southerland W. M., Toghrol F. Sulfite oxidase activity in Thiobacillus novellus. J Bacteriol. 1983 Nov;156(2):941–944. doi: 10.1128/jb.156.2.941-944.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Munakata O., Tano T., Imai K. Role of a Ferric Ion-Reducing System in Sulfur Oxidation of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1985 Jun;49(6):1401–1406. doi: 10.1128/aem.49.6.1401-1406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Domatsu C., Tano T., Imai K. Role of Ferrous Ions in Synthetic Cobaltous Sulfide Leaching of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1984 Sep;48(3):461–467. doi: 10.1128/aem.48.3.461-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Katagiri T., Moriyama M., Zhèn Y. L., Inagaki K., Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988 Jan;54(1):153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., Mizunashi W., Inagaki K., Tano T. Purification and some properties of sulfur:ferric ion oxidoreductase from Thiobacillus ferrooxidans. J Bacteriol. 1987 Nov;169(11):4916–4922. doi: 10.1128/jb.169.11.4916-4922.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio T., White K. J., Shute E., Choate D., Blake R. C. Existence of a hydrogen sulfide:ferric ion oxidoreductase in iron-oxidizing bacteria. Appl Environ Microbiol. 1992 Jan;58(1):431–433. doi: 10.1128/aem.58.1.431-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghrol F., Southerland W. M. Purification of Thiobacillus novellus sulfite oxidase. Evidence for the presence of heme and molybdenum. J Biol Chem. 1983 Jun 10;258(11):6762–6766. [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G. The sulfite oxidase of Thiobacillus ferrooxidans (Ferrobacillus ferrooxidans). Can J Biochem. 1971 Oct;49(10):1125–1130. doi: 10.1139/o71-162. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]