Abstract
Mycoplasmas are wall-less prokaryotes phylogenetically related to gram-positive bacteria. In order to investigate DNA recombination in these organisms, we have cloned the recA gene from the mycoplasma Acholeplasma laidlawii. DNA sequence data indicate extensive homology between the A. laidlawii recA gene and recA genes from other bacteria, particularly Bacillus subtilis. The recA sequences from three A. laidlawii strains (strains JA1, K2, and 8195) were compared, and surprisingly, the gene from A. laidlawii 8195 was found to contain a nonsense mutation that results in truncation of 36 amino acids from the carboxyl terminus of the RecA protein. By using sensitivity to UV irradiation as a measure of DNA repair, strain 8195 had an apparent RecA- phenotype. When carried on a multicopy plasmid, the wild-type A. laidlawii recA gene was detrimental to growth of Escherichia coli, perhaps because of improper regulation of the RecA protein.
Full text
PDF

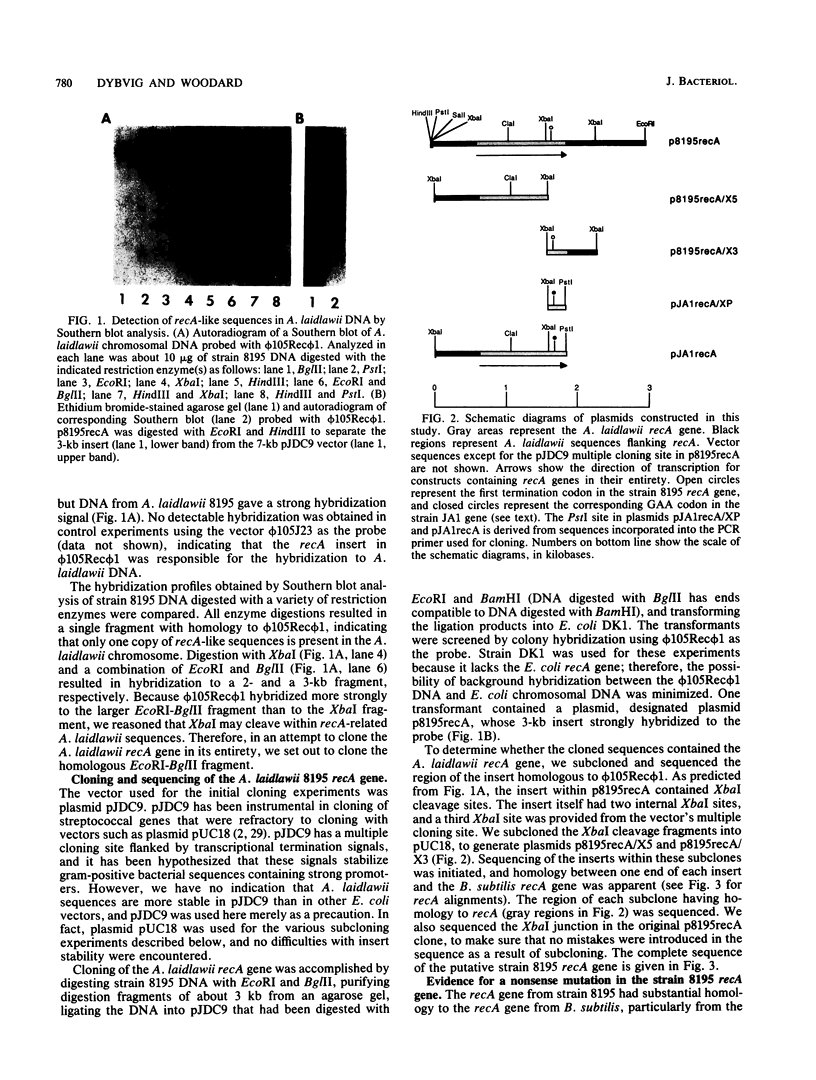
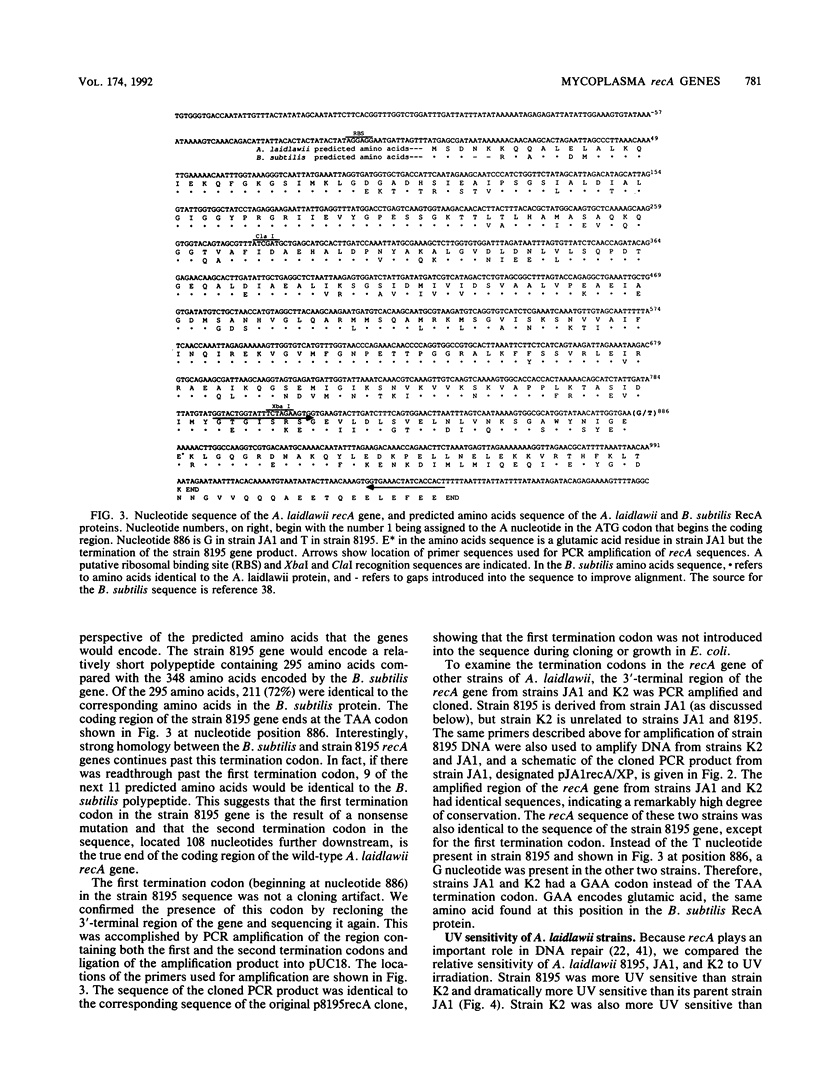
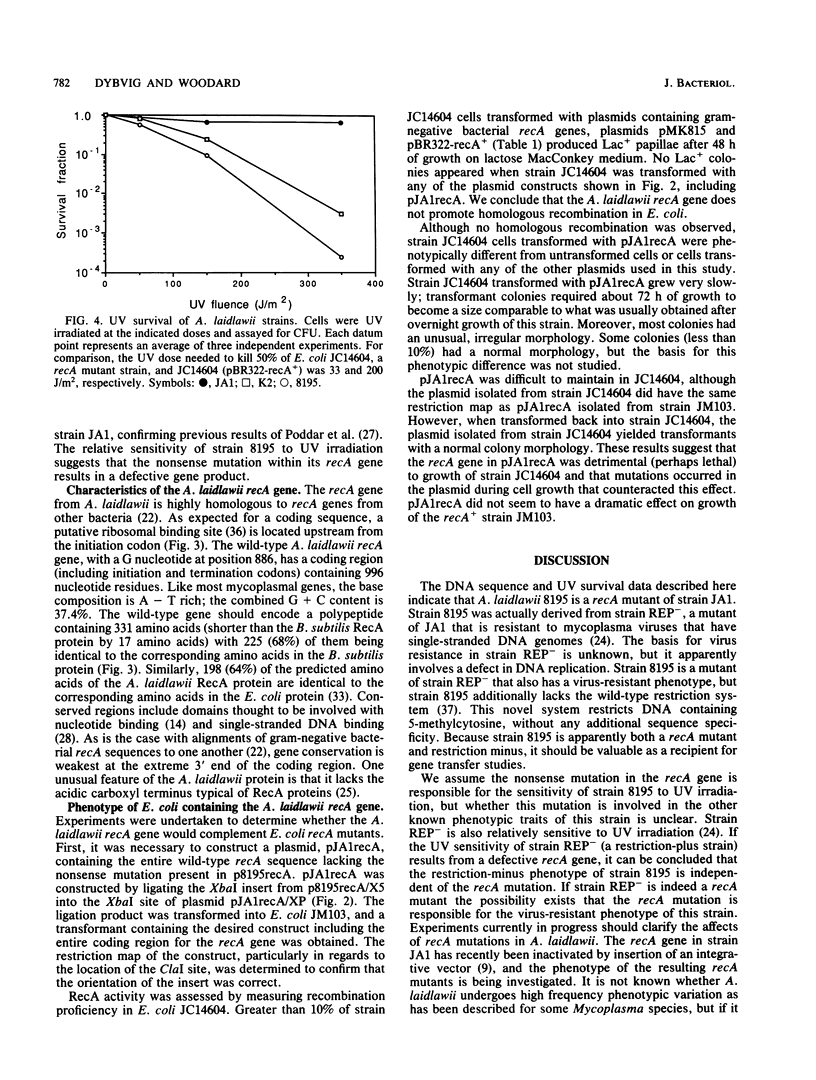


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Morrison D. A. Cloning of Streptococcus pneumoniae DNA fragments in Escherichia coli requires vectors protected by strong transcriptional terminators. Gene. 1987;55(2-3):179–187. doi: 10.1016/0378-1119(87)90278-2. [DOI] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Prevalence of novel repeat sequences in and around the P1 operon in the genome of Mycoplasma pneumoniae. Gene. 1990 Mar 1;87(1):91–96. doi: 10.1016/0378-1119(90)90498-g. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988 Jul;20(1):33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Cassell G. H. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science. 1987 Mar 13;235(4794):1392–1394. doi: 10.1126/science.3029869. [DOI] [PubMed] [Google Scholar]
- Dybvig K. Mycoplasmal genetics. Annu Rev Microbiol. 1990;44:81–104. doi: 10.1146/annurev.mi.44.100190.000501. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Simecka J. W., Watson H. L., Cassell G. H. High-frequency variation in Mycoplasma pulmonis colony size. J Bacteriol. 1989 Sep;171(9):5165–5168. doi: 10.1128/jb.171.9.5165-5168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Efficient Bacillus subtilis cloning system using bacteriophage vector phi 105J9. J Gen Microbiol. 1984 Oct;130(10):2615–2628. doi: 10.1099/00221287-130-10-2615. [DOI] [PubMed] [Google Scholar]
- Flock J. I. Deletion mutants of temperate Bacillus subtilis bacteriophage phi105. Mol Gen Genet. 1977 Oct 24;155(3):241–247. doi: 10.1007/BF00272803. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Nucleotide binding by a 24-residue peptide from the RecA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9289–9293. doi: 10.1073/pnas.83.24.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnit D. M. Escherichia coli recA deletion strains that are highly competent for transformation and for in vivo phage packaging. Gene. 1989 Oct 30;82(2):313–315. doi: 10.1016/0378-1119(89)90056-5. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Infection of Acholeplasma laidlawii by MVL51 virus. Virology. 1973 Sep;55(1):118–126. doi: 10.1016/s0042-6822(73)81013-x. [DOI] [PubMed] [Google Scholar]
- Mahairas G. G., Minion F. C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. J Bacteriol. 1989 Apr;171(4):1775–1780. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Marrero R., Yasbin R. E. Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in B. subtilis. J Bacteriol. 1988 Jan;170(1):335–344. doi: 10.1128/jb.170.1.335-344.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Nowak J. A., Das J., Maniloff J. Characterization of an Acholeplasma laidlawii variant with a REP- phenotype. J Bacteriol. 1976 Aug;127(2):832–836. doi: 10.1128/jb.127.2.832-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Ogawa T. General recombination: functions and structure of RecA protein. Adv Biophys. 1986;21:135–148. doi: 10.1016/0065-227x(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Ogawa T. Regulation in repressor inactivation by RecA protein. Adv Biophys. 1990;26:33–49. doi: 10.1016/0065-227x(90)90006-f. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Cadden S. P., Das J., Maniloff J. Heterogeneous progeny viruses are produced by a budding enveloped phage. Intervirology. 1985;23(4):208–221. doi: 10.1159/000149607. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Chiu W. Sequence comparison of single-stranded DNA binding proteins and its structural implications. J Mol Biol. 1987 Feb 5;193(3):579–584. doi: 10.1016/0022-2836(87)90268-3. [DOI] [PubMed] [Google Scholar]
- Rhee D. K., Morrison D. A. Genetic transformation in Streptococcus pneumoniae: molecular cloning and characterization of recP, a gene required for genetic recombination. J Bacteriol. 1988 Feb;170(2):630–637. doi: 10.1128/jb.170.2.630-637.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988 Sep;52(3):327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Nowak J. A., Maniloff J. Mycoplasma restriction: identification of a new type of restriction specificity for DNA containing 5-methylcytosine. J Bacteriol. 1986 Jan;165(1):219–225. doi: 10.1128/jb.165.1.219-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranathan M. C., Bayles K. W., Yasbin R. E. The nucleotide sequence of the recE+ gene of Bacillus subtilis. Nucleic Acids Res. 1990 Jul 25;18(14):4249–4249. doi: 10.1093/nar/18.14.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Baseman J. B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988 Dec;56(12):3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]