Abstract
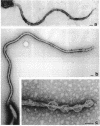
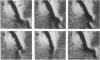
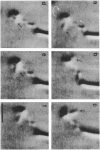
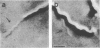
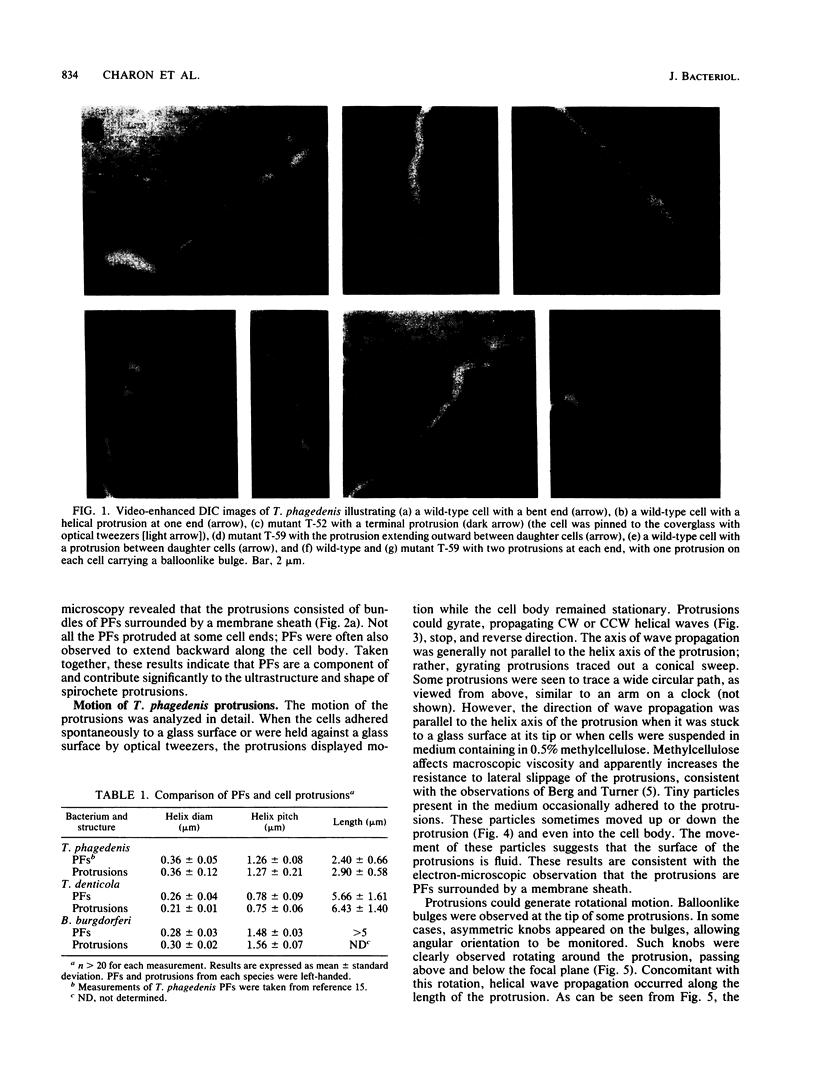
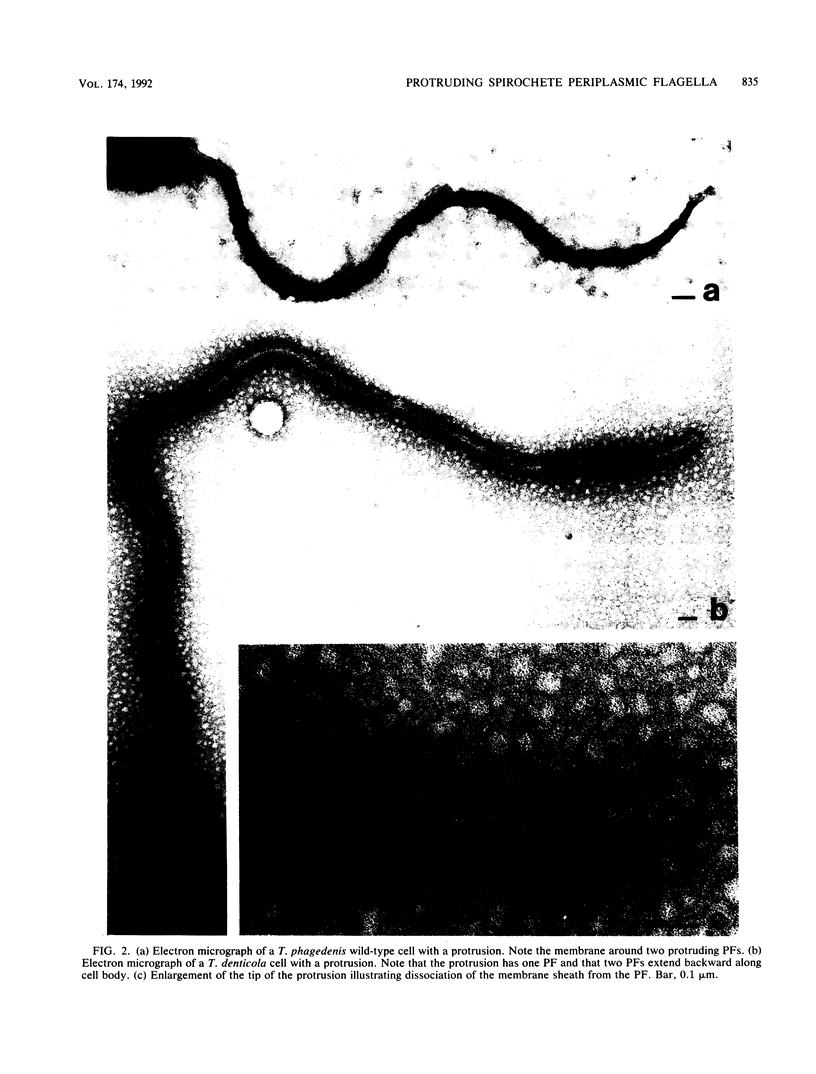
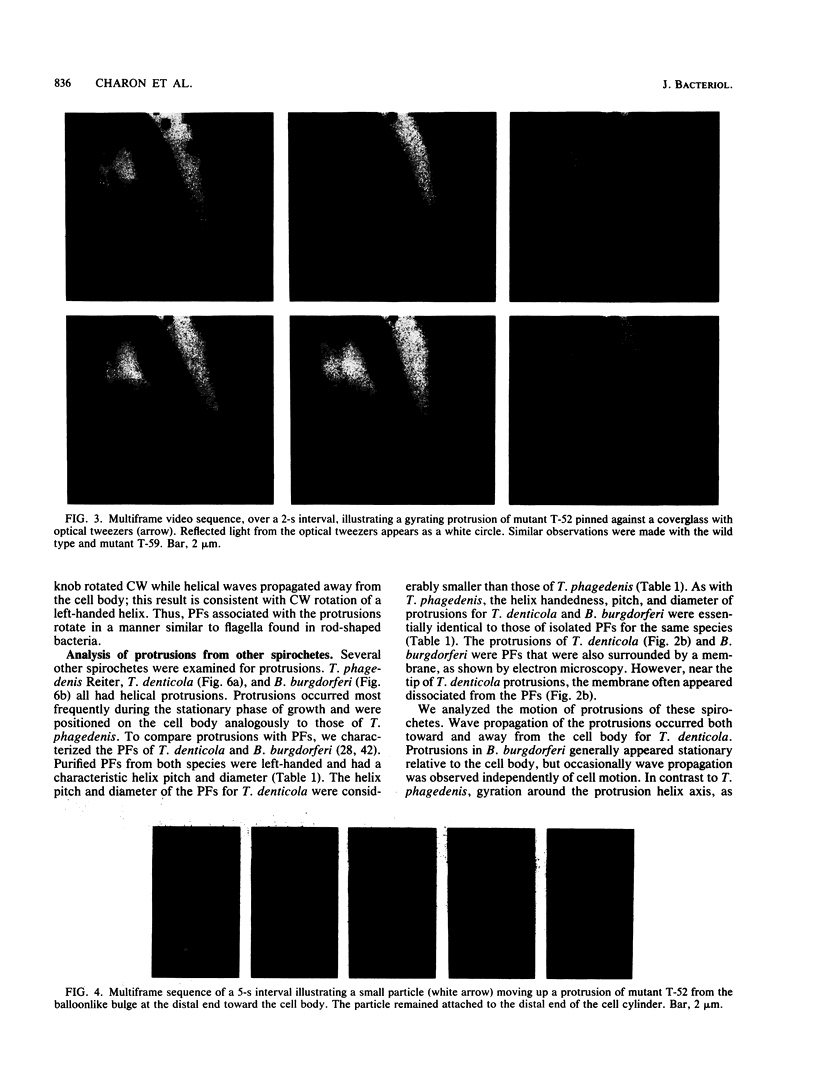
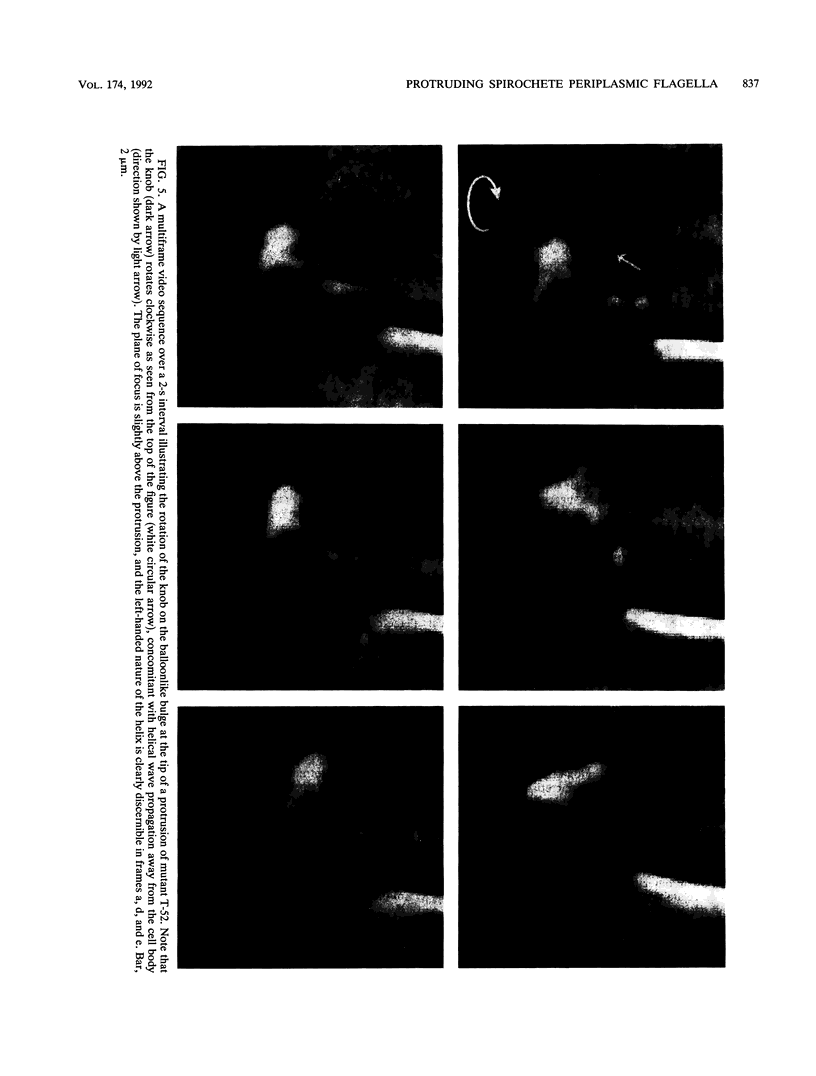
We recently characterized the three-dimensional shape of Treponema phagedenis periplasmic flagella (PFs). In the course of these studies, we observed protrusions on swimming cells that resembled PFs. Here we present a detailed characterization of the shape, structure, and motion of these protrusions. Although protrusion formation occurred primarily in wild-type cells during the stationary phase, a large fraction of exponential-phase cells of cell cylinder helicity mutants (greater than 90% of mutant T-52) had protrusions. These results suggest that cells bearing protrusions can still participate in cell division. T. phagedenis protrusions had the identical helix handedness, pitch, and diameter to those of purified PFs. Protrusions were not present on mutants unable to synthesize PFs, but were present in all motile revertants which regained PFs. These results, taken together with electron microscope observations, suggest that protrusions consist of PFs surrounded by an outer membrane sheath. To analyze protrusion movements, we held cells against a coverglass surface with optical tweezers and observed the motion of protrusions by video-enhanced differential interference contrast light microscopy. Protrusions were found to gyrate in both clockwise and counterclockwise directions, and direct evidence was obtained that protrusions rotate. Protrusions were also observed on Treponema denticola and Borrelia burgdorferi. These were also left-handed and had the same helix handedness, pitch, and diameter as purified PFs from their respective species. The PFs from T. denticola had a helix diameter of 0.26 microns and a helix pitch of 0.78 micron; PFs from B. burgdorferi had a helix diameter of 0.28 micron and a helix pitch of 1.48 microns. Protrusions from these spirochete species had similar structures and motion to those of T. phagedenis. Our results present direct evidence that PFs rotate and support previously proposed models of spirochete motility.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Blair D. F. The bacterial flagellar motor. Semin Cell Biol. 1990 Apr;1(2):75–85. [PubMed] [Google Scholar]
- Blanco D. R., Radolf J. D., Lovett M. A., Miller J. N. The antigenic interrelationship between the endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum Nichols strain. I. Treponemicidal activity of cross-reactive endoflagellar antibodies against T. pallidum. J Immunol. 1986 Nov 1;137(9):2973–2979. [PubMed] [Google Scholar]
- Block S. M., Blair D. F., Berg H. C. Compliance of bacterial flagella measured with optical tweezers. Nature. 1989 Apr 6;338(6215):514–518. doi: 10.1038/338514a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Fahrner K. A., Berg H. C. Visualization of bacterial flagella by video-enhanced light microscopy. J Bacteriol. 1991 Jan;173(2):933–936. doi: 10.1128/jb.173.2.933-936.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Carleton O., Charon N. W., Allender P., O'Brien S. Helix handedness of Leptospira interrogans as determined by scanning electron microscopy. J Bacteriol. 1979 Mar;137(3):1413–1416. doi: 10.1128/jb.137.3.1413-1416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Daughtry G. R., McCuskey R. S., Franz G. N. Microcinematographic analysis of tethered Leptospira illini. J Bacteriol. 1984 Dec;160(3):1067–1073. doi: 10.1128/jb.160.3.1067-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Curci K., Limberger R. J. The bent-end morphology of Treponema phagedenis is associated with short, left-handed, periplasmic flagella. J Bacteriol. 1991 Aug;173(15):4820–4826. doi: 10.1128/jb.173.15.4820-4826.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Lawrence C. W., O'Brien S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7166–7170. doi: 10.1073/pnas.78.11.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne A., Bailey M. J., Penn C. W. Analysis of sheath and core structures of the axial filament of Treponema pallidum. J Gen Microbiol. 1987 Jun;133(6):1397–1407. doi: 10.1099/00221287-133-6-1397. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J Clin Invest. 1989 Jul;84(1):322–330. doi: 10.1172/JCI114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Chemotaxis mutants of Spirochaeta aurantia. J Bacteriol. 1989 Jan;171(1):606–611. doi: 10.1128/jb.171.1.606-611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Motility and chemotaxis of Spirochaeta aurantia: computer-assisted motion analysis. J Bacteriol. 1988 Apr;170(4):1768–1774. doi: 10.1128/jb.170.4.1768-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Motility of the spirochete Leptospira. Cell Motil Cytoskeleton. 1988;9(2):101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Multiple-exposure photographic analysis of a motile spirochete. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4895–4899. doi: 10.1073/pnas.87.13.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp E. G., Scott D. B., Wyckoff R. W. Morphologic Characteristics of Certain Cultured Strains of Oral Spirochetes and Treponema pallidum as Revealed by the Electron Microscope. J Bacteriol. 1948 Dec;56(6):755–769. doi: 10.1128/jb.56.6.755-769.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Rifampin as a selective agent for isolation of oral spirochetes. J Clin Microbiol. 1980 Dec;12(6):792–795. doi: 10.1128/jcm.12.6.792-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Treponema phagedenis has at least two proteins residing together on its periplasmic flagella. J Bacteriol. 1986 Apr;166(1):105–112. doi: 10.1128/jb.166.1.105-112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON H. E., RAKE G., ROSE N. R. Electron microscope studies of treponemes. III. Flagella. Am J Syph Gonorrhea Vener Dis. 1951 Nov;35(6):503–516. [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E., Weisburg W. G., Tordoff L. A., Fraser G. J., Hespell R. B., Stanton T. B., Zablen L., Mandelco L., Woese C. R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991 Oct;173(19):6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrantonio F., Noble P. B., Amsel R., Chan E. C. Locomotory characteristics of Treponema denticola. Can J Microbiol. 1988 Jun;34(6):748–752. doi: 10.1139/m88-127. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]