Abstract
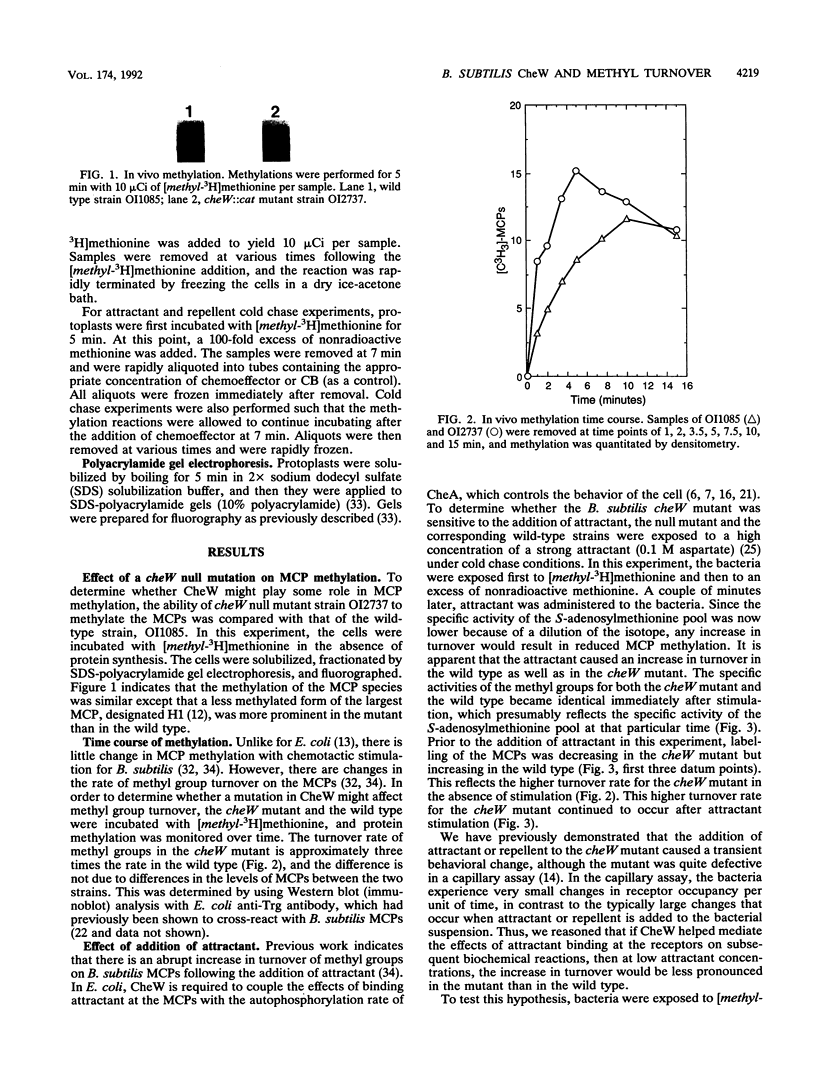
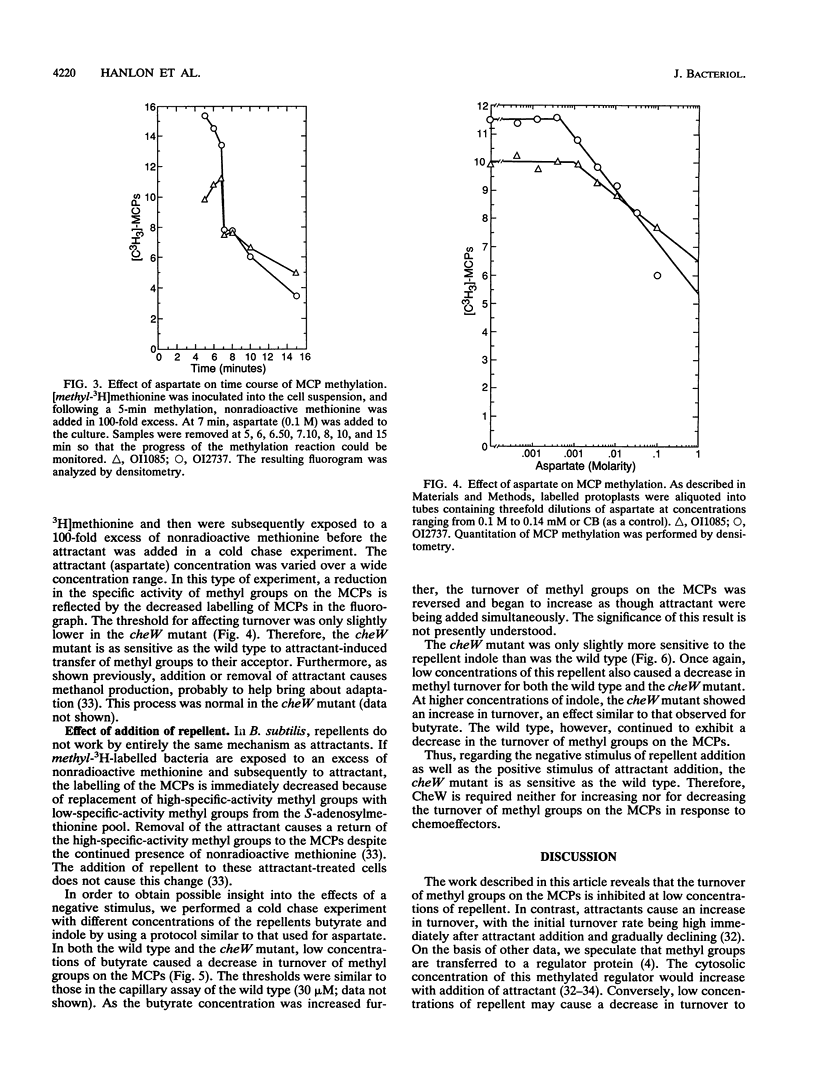
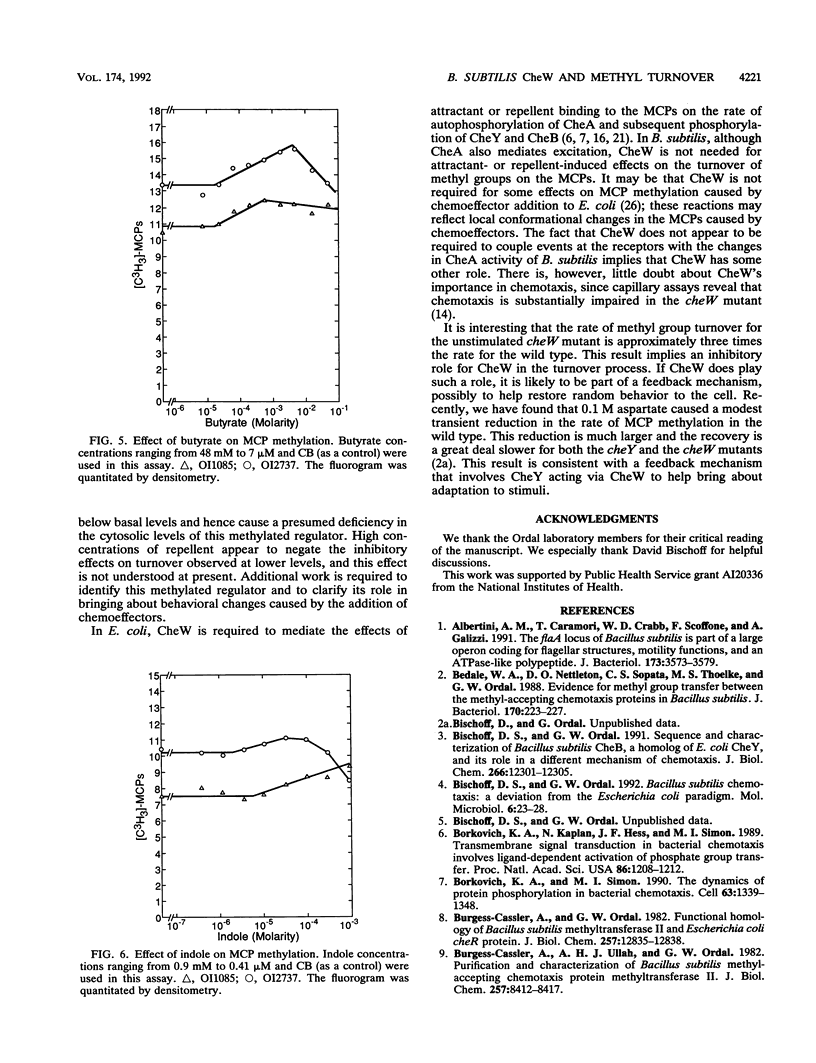
The ability of attractants and repellents to affect the turnover of methyl groups on the methyl-accepting chemotaxis proteins (MCPs) was examined for Bacillus subtilis. Attractants were found to cause an increase in the turnover of methyl groups esterified to the MCPs, while repellents caused a decrease. These reactions do not require CheW. However, a cheW null mutant exhibits enhanced turnover in unstimulated cells. Assuming that the turnover of methyl groups on the MCPs reflects a change in the activity of CheA, these results suggest that the activation of CheA via chemoeffector binding at the receptor does not require CheW.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Caramori T., Crabb W. D., Scoffone F., Galizzi A. The flaA locus of Bacillus subtilis is part of a large operon coding for flagellar structures, motility functions, and an ATPase-like polypeptide. J Bacteriol. 1991 Jun;173(11):3573–3579. doi: 10.1128/jb.173.11.3573-3579.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedale W. A., Nettleton D. O., Sopata C. S., Thoelke M. S., Ordal G. W. Evidence for methyl group transfer between the methyl-accepting chemotaxis proteins in Bacillus subtilis. J Bacteriol. 1988 Jan;170(1):223–227. doi: 10.1128/jb.170.1.223-227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff D. S., Ordal G. W. Bacillus subtilis chemotaxis: a deviation from the Escherichia coli paradigm. Mol Microbiol. 1992 Jan;6(1):23–28. doi: 10.1111/j.1365-2958.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Bischoff D. S., Ordal G. W. Sequence and characterization of Bacillus subtilis CheB, a homolog of Escherichia coli CheY, and its role in a different mechanism of chemotaxis. J Biol Chem. 1991 Jul 5;266(19):12301–12305. [PubMed] [Google Scholar]
- Borkovich K. A., Kaplan N., Hess J. F., Simon M. I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Simon M. I. The dynamics of protein phosphorylation in bacterial chemotaxis. Cell. 1990 Dec 21;63(6):1339–1348. doi: 10.1016/0092-8674(90)90429-i. [DOI] [PubMed] [Google Scholar]
- Burgess-Cassler A., Ordal G. W. Functional homology of Bacillus subtilis methyltransferase II and Escherichia coli cheR protein. J Biol Chem. 1982 Nov 10;257(21):12835–12838. [PubMed] [Google Scholar]
- Burgess-Cassler A., Ullah A. H., Ordal G. W. Purification and characterization of Bacillus subtilis methyl-accepting chemotaxis protein methyltransferase II. J Biol Chem. 1982 Jul 25;257(14):8412–8417. [PubMed] [Google Scholar]
- Fuhrer D. K., Ordal G. W. Bacillus subtilis CheN, a homolog of CheA, the central regulator of chemotaxis in Escherichia coli. J Bacteriol. 1991 Dec;173(23):7443–7448. doi: 10.1128/jb.173.23.7443-7448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. J., Nettleton D. O., Ordal G. W. Purification and characterization of chemotactic methylesterase from Bacillus subtilis. Biochemistry. 1984 Feb 14;23(4):675–680. doi: 10.1021/bi00299a014. [DOI] [PubMed] [Google Scholar]
- Goldman D. J., Nettleton D. O., Ordal G. W. Purification and characterization of chemotactic methylesterase from Bacillus subtilis. Biochemistry. 1984 Feb 14;23(4):675–680. doi: 10.1021/bi00299a014. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon D. W., Márquez-Magaña L. M., Carpenter P. B., Chamberlin M. J., Ordal G. W. Sequence and characterization of Bacillus subtilis CheW. J Biol Chem. 1992 Jun 15;267(17):12055–12060. [PubMed] [Google Scholar]
- Kihara M., Homma M., Kutsukake K., Macnab R. M. Flagellar switch of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989 Jun;171(6):3247–3257. doi: 10.1128/jb.171.6.3247-3257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Role of CheW protein in coupling membrane receptors to the intracellular signaling system of bacterial chemotaxis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Stock J. Phosphorylation of an N-terminal regulatory domain activates the CheB methylesterase in bacterial chemotaxis. J Biol Chem. 1989 Oct 15;264(29):17337–17342. [PubMed] [Google Scholar]
- Malakooti J., Komeda Y., Matsumura P. DNA sequence analysis, gene product identification, and localization of flagellar motor components of Escherichia coli. J Bacteriol. 1989 May;171(5):2728–2734. doi: 10.1128/jb.171.5.2728-2734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton D. O., Ordal G. W. Functional homology of chemotactic methylesterases from Bacillus subtilis and Escherichia coli. J Bacteriol. 1989 Jan;171(1):120–123. doi: 10.1128/jb.171.1.120-123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J., Ninfa E. G., Lupas A. N., Stock A., Magasanik B., Stock J. Crosstalk between bacterial chemotaxis signal transduction proteins and regulators of transcription of the Ntr regulon: evidence that nitrogen assimilation and chemotaxis are controlled by a common phosphotransfer mechanism. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5492–5496. doi: 10.1073/pnas.85.15.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa E. G., Stock A., Mowbray S., Stock J. Reconstitution of the bacterial chemotaxis signal transduction system from purified components. J Biol Chem. 1991 May 25;266(15):9764–9770. [PubMed] [Google Scholar]
- Nowlin D. M., Nettleton D. O., Ordal G. W., Hazelbauer G. L. Chemotactic transducer proteins of Escherichia coli exhibit homology with methyl-accepting proteins from distantly related bacteria. J Bacteriol. 1985 Jul;163(1):262–266. doi: 10.1128/jb.163.1.262-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Hess J. F., Simon M. I. Mutants defective in bacterial chemotaxis show modified protein phosphorylation. Cell. 1988 Apr 8;53(1):89–96. doi: 10.1016/0092-8674(88)90490-4. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Nettleton D. O., Hoch J. A. Genetics of Bacillus subtilis chemotaxis: isolation and mapping of mutations and cloning of chemotaxis genes. J Bacteriol. 1983 Jun;154(3):1088–1097. doi: 10.1128/jb.154.3.1088-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Gibson K. J. Amino acid chemoreceptors of Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):156–165. doi: 10.1128/jb.129.1.156-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Behavioral genetics in bacteria. Annu Rev Genet. 1977;11:397–414. doi: 10.1146/annurev.ge.11.120177.002145. [DOI] [PubMed] [Google Scholar]
- Ravid S., Matsumura P., Eisenbach M. Restoration of flagellar clockwise rotation in bacterial envelopes by insertion of the chemotaxis protein CheY. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7157–7161. doi: 10.1073/pnas.83.19.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Rowsell E. H., Shioi J., Taylor B. L. Identification of a site of ATP requirement for signal processing in bacterial chemotaxis. J Bacteriol. 1988 Jun;170(6):2698–2704. doi: 10.1128/jb.170.6.2698-2704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979 Jul 26;280(5720):279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Thoelke M. S., Bedale W. A., Nettleton D. O., Ordal G. W. Evidence for an intermediate methyl-acceptor for chemotaxis in Bacillus subtilis. J Biol Chem. 1987 Feb 25;262(6):2811–2816. [PubMed] [Google Scholar]
- Thoelke M. S., Casper J. M., Ordal G. W. Methyl group turnover on methyl-accepting chemotaxis proteins during chemotaxis by Bacillus subtilis. J Biol Chem. 1990 Feb 5;265(4):1928–1932. [PubMed] [Google Scholar]
- Thoelke M. S., Kirby J. R., Ordal G. W. Novel methyl transfer during chemotaxis in Bacillus subtilis. Biochemistry. 1989 Jun 27;28(13):5585–5589. doi: 10.1021/bi00439a037. [DOI] [PubMed] [Google Scholar]
- Thoelke M. S., Parker H. M., Ordal E. A., Ordal G. W. Rapid attractant-induced changes in methylation of methyl-accepting chemotaxis proteins in Bacillus subtilis. Biochemistry. 1988 Nov 1;27(22):8453–8457. doi: 10.1021/bi00422a024. [DOI] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Aizawa S., Kihara M., Isomura M., Jones C. J., Macnab R. M. Genetic evidence for a switching and energy-transducing complex in the flagellar motor of Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1172–1179. doi: 10.1128/jb.168.3.1172-1179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi A. R., Bischoff D. S., Ordal G. W. Nucleotide sequence and characterization of a Bacillus subtilis gene encoding a flagellar switch protein. J Bacteriol. 1991 Jan;173(2):710–719. doi: 10.1128/jb.173.2.710-719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]




