Abstract
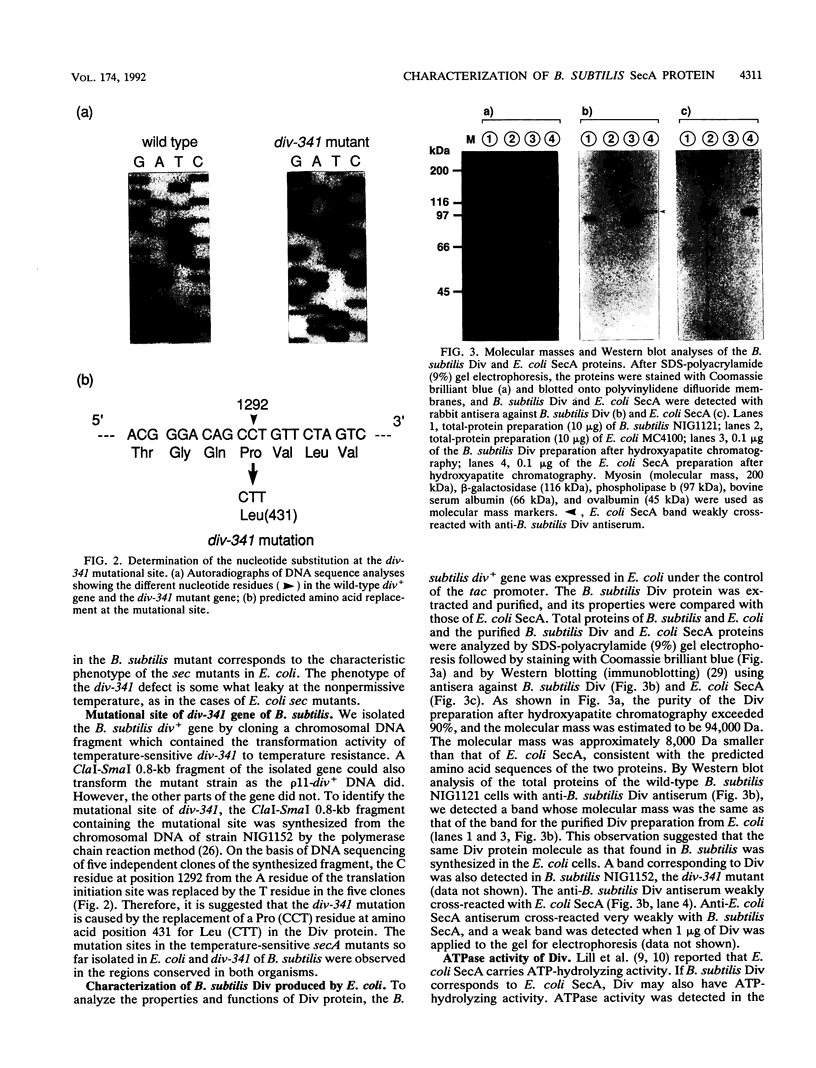
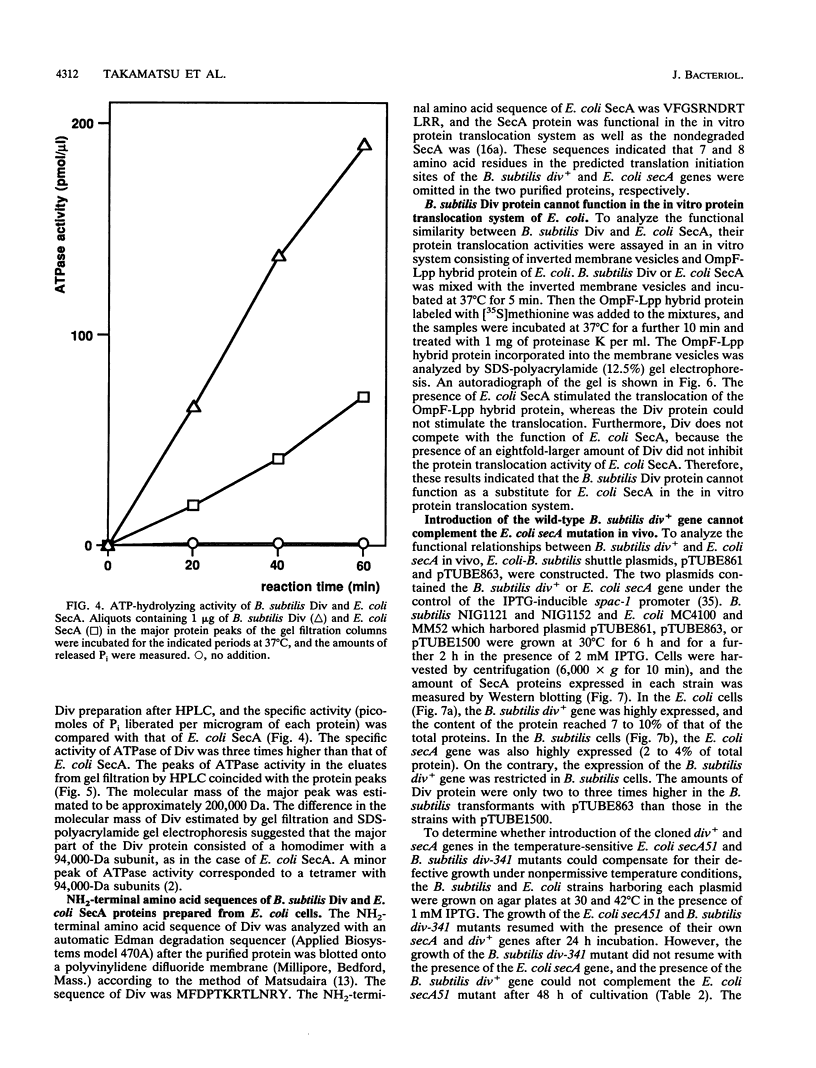
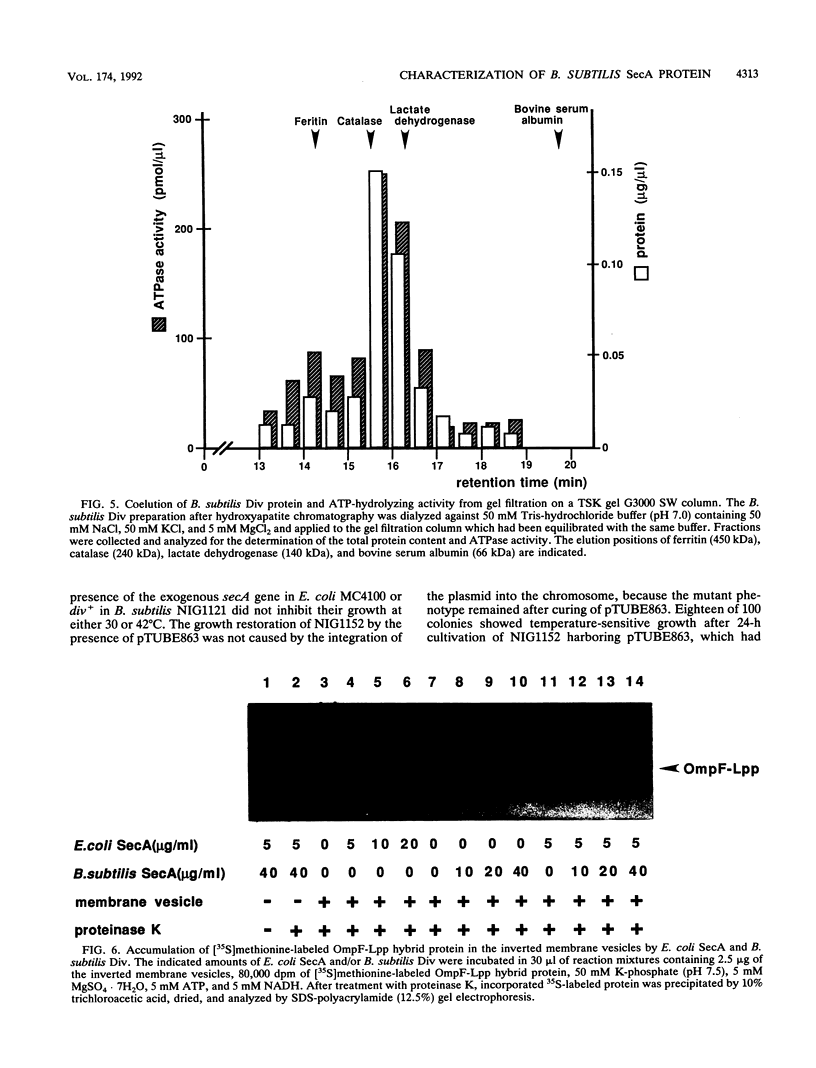
The putative amino acid sequence from the wild-type Bacillus subtilis div+ gene, which complements the temperature-sensitive div-341 mutation, shares a 50% identity with the sequence from Escherichia coli secA (Y. Sadaie, H. Takamatsu, K. Nakamura, and K. Yamane, Gene 98:101-105, 1991). The B. subtilis div-341 mutant accumulated the precursor proteins of alpha-amylase and beta-lactamase at 45 degrees C as in the case of sec mutants of E. coli. The div-341 mutation is a transition mutation causing an amino acid replacement from Pro to Leu at residue 431 of the putative amino acid sequence. The B. subtilis div+ gene was overexpressed in E. coli under the control of the tac promoter, and its product was purified to homogeneity. The Div protein consists of a homodimer of 94-kDa subunits which possesses ATPase activity, and the first 7 amino acids of the putative Div protein were found to be subjected to limited proteolysis in the purified protein. The antiserum against B. subtilis Div weakly cross-reacted with E. coli SecA. On the other hand, B. subtilis Div could not replace E. coli SecA in an E. coli in vitro protein translocation system. The temperature-sensitive growth of the E. coli secA mutant could not be restored by the introduction of B. subtilis div+, which is expressed under the control of the spac-1 promoter, and vice versa. The B. subtilis div+ gene is the B. subtilis counterpart of E. coli secA, and we propose that the div+ gene be referred to as B. subtilis secA, although Div did not function in the protein translocation system of E. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita M., Sasaki S., Matsuyama S., Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem. 1990 May 15;265(14):8164–8169. [PubMed] [Google Scholar]
- Akita M., Shinkai A., Matsuyama S., Mizushima S. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem Biophys Res Commun. 1991 Jan 15;174(1):211–216. doi: 10.1016/0006-291x(91)90507-4. [DOI] [PubMed] [Google Scholar]
- Cabelli R. J., Chen L., Tai P. C., Oliver D. B. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988 Nov 18;55(4):683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- Henner D. J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- Ishiwa H., Tsuchida N. New shuttle vectors for Escherichia coli and Bacillus subtilis. I. Construction and characterization of plasmid pHY460 with twelve unique cloning sites. Gene. 1984 Dec;32(1-2):129–134. doi: 10.1016/0378-1119(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Matsuyama S., Sasaki S., Akita M., Mizushima S. SecA protein is directly involved in protein secretion in Escherichia coli. FEBS Lett. 1989 Jan 2;242(2):431–434. doi: 10.1016/0014-5793(89)80516-2. [DOI] [PubMed] [Google Scholar]
- Kimura E., Akita M., Matsuyama S., Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991 Apr 5;266(10):6600–6606. [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Liss L. R., Oliver D. B. Effects of secA mutations on the synthesis and secretion of proteins in Escherichia coli. Evidence for a major export system for cell envelope proteins. J Biol Chem. 1986 Feb 15;261(5):2299–2303. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Matsuzaki H., Yamane K., Maruo B. Hybrid alpha-amylases produced by transformants of Bacillus subtilis. II. Immunological and chemical properties of alpha-amylases produced by the parental strains and the transformants. Biochim Biophys Acta. 1974 Sep 13;365(1):248–258. doi: 10.1016/0005-2795(74)90269-4. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan V. System for secretion of heterologous proteins in Bacillus subtilis. Methods Enzymol. 1990;185:214–223. doi: 10.1016/0076-6879(90)85021-f. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Takamatsu H., Akiyama Y., Ito K., Yamane K. Complementation of the protein transport defect of an Escherichia coli secY mutant (secY24) by Bacillus subtilis secY homologue. FEBS Lett. 1990 Oct 29;273(1-2):75–78. doi: 10.1016/0014-5793(90)81054-r. [DOI] [PubMed] [Google Scholar]
- Ohmura K., Nakamura K., Yamazaki H., Shiroza T., Yamane K., Jigami Y., Tanaka H., Yoda K., Yamasaki M., Tamura G. Length and structural effect of signal peptides derived from Bacillus subtilis alpha-amylase on secretion of Escherichia coli beta-lactamase in B. subtilis cells. Nucleic Acids Res. 1984 Jul 11;12(13):5307–5319. doi: 10.1093/nar/12.13.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J Bacteriol. 1982 May;150(2):686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhoff B., Klein M., Spies M., Freudl R. Identification of a gene fragment which codes for the 364 amino-terminal amino acid residues of a SecA homologue from Bacillus subtilis: further evidence for the conservation of the protein export apparatus in gram-positive and gram-negative bacteria. Mol Gen Genet. 1991 Sep;228(3):417–423. doi: 10.1007/BF00260635. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Bacillus subtilis gene involved in cell division, sporulation, and exoenzyme secretion. J Bacteriol. 1985 Aug;163(2):648–653. doi: 10.1128/jb.163.2.648-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y. Molecular cloning of a Bacillus subtilis gene involved in cell division, sporulation, and exoenzyme secretion. Jpn J Genet. 1989 Apr;64(2):111–119. doi: 10.1266/jjg.64.111. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Takamatsu H., Nakamura K., Yamane K. Sequencing reveals similarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli secA gene. Gene. 1991 Feb 1;98(1):101–105. doi: 10.1016/0378-1119(91)90110-w. [DOI] [PubMed] [Google Scholar]
- Schmidt M. G., Rollo E. E., Grodberg J., Oliver D. B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tokuda H., Mizushima S. Proton motive force-dependent and -independent protein translocation revealed by an efficient in vitro assay system of Escherichia coli. J Biol Chem. 1989 Jan 25;264(3):1723–1728. [PubMed] [Google Scholar]
- Yamane K., Hirata Y., Furusato T., Yamazaki H., Nakayama A. Changes in the properties and molecular weights of Bacillus subtilis M-type and N-type alpha-amylases resulting from a spontaneous deletion. J Biochem. 1984 Dec;96(6):1849–1858. doi: 10.1093/oxfordjournals.jbchem.a135019. [DOI] [PubMed] [Google Scholar]
- Yamane K., Ichihara S., Mizushima S. In vitro translocation of protein across Escherichia coli membrane vesicles requires both the proton motive force and ATP. J Biol Chem. 1987 Feb 15;262(5):2358–2362. [PubMed] [Google Scholar]
- Yamane K., Matsuyama S., Mizushima S. Efficient in vitro translocation into Escherichia coli membrane vesicles of a protein carrying an uncleavable signal peptide. Characterization of the translocation process. J Biol Chem. 1988 Apr 15;263(11):5368–5372. [PubMed] [Google Scholar]
- Yamane K., Mizushima S. Introduction of basic amino acid residues after the signal peptide inhibits protein translocation across the cytoplasmic membrane of Escherichia coli. Relation to the orientation of membrane proteins. J Biol Chem. 1988 Dec 25;263(36):19690–19696. [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]







