Abstract
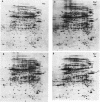
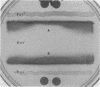
Fur is an important regulatory protein known to function in the presence of iron as a repressor of iron-controlled genes. It was recently discovered that Fur is also essential to Salmonella typhimurium for mounting an adaptive acid tolerance response (J. W. Foster, J. Bacteriol 173:6896-6902, 1991). Because little is known about the effect of Fur on the physiology of this enteric pathogen, a systematic two-dimensional polyacrylamide gel electrophoresis (PAGE) analysis was conducted to identify proteins whose synthesis is linked to iron levels. Mutations in the fur locus were identified and used to classify which proteins are controlled by Fur. Thirty-six proteins were overtly affected by iron availability, most of which were clearly under the control of Fur. Although most of the Fur-dependent proteins were under negative control, a significant portion (15 of 34) appeared to be under a form of positive control. Nine of the positively controlled proteins required Fur and iron for expression. However, Fur lacking iron was also required for the induction of six gene products. Surprisingly, not all iron-regulated proteins were controlled by Fur and not all Fur-dependent proteins were obviously regulated by iron status. Because fur mutants fail to mount an effective acid tolerance response, we made a comparative two-dimensional PAGE analysis of 100 total acid- and iron-regulated gene products. Production of most of these proteins was regulated by only one of the two stresses, yet a clear subset of seven genes were influenced by both acid and iron and were also controlled by fur. These proteins were also members of the acid tolerance response modulon. Consistent with the fur effect on pH-regulated protein synthesis, fur mutants lacked the inducible pH homeostasis system associated with the acid tolerance response. The results provide further evidence that Fur has an extensive impact on gene expression and cellular physiology and suggest an explanation for the acid-sensitive nature of fur mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell. 1983 Jan;32(1):141–149. doi: 10.1016/0092-8674(83)90504-4. [DOI] [PubMed] [Google Scholar]
- Aiba H., Nakamura T., Mitani H., Mori H. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3329–3332. doi: 10.1002/j.1460-2075.1985.tb04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by cAMP-cAMP receptor protein. J Biol Chem. 1985 Mar 10;260(5):3063–3070. [PubMed] [Google Scholar]
- Aliabadi Z., Park Y. K., Slonczewski J. L., Foster J. W. Novel regulatory loci controlling oxygen- and pH-regulated gene expression in Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):842–851. doi: 10.1128/jb.170.2.842-851.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985 Nov;50(2):392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. L., Rothfield L. I. Genetic and physiological regulation of intrinsic proteins of the outer membrane of Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):498–504. doi: 10.1128/jb.127.1.498-504.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J. F., Bennett R. L., Rothfield L. I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978 Sep;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991 Nov;173(21):6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garges S., Adhya S. Cyclic AMP-induced conformational change of cyclic AMP receptor protein (CRP): intragenic suppressors of cyclic AMP-independent CRP mutations. J Bacteriol. 1988 Apr;170(4):1417–1422. doi: 10.1128/jb.170.4.1417-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1125–1129. doi: 10.1073/pnas.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Hennecke H. Regulation of bacterial gene expression by metal-protein complexes. Mol Microbiol. 1990 Oct;4(10):1621–1628. doi: 10.1111/j.1365-2958.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Holley E. A., Foster J. W. Bacteriophage P22 as a vector for Mu mutagenesis in Salmonella typhimurium: isolation of nad-lac and pnc-lac gene fusions. J Bacteriol. 1982 Nov;152(2):959–962. doi: 10.1128/jb.152.2.959-962.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Park Y. K., Tirgari S., Gonzalez T., Foster J. W. Identification and characterization of starvation-regulated genetic loci in Salmonella typhimurium by using Mu d-directed lacZ operon fusions. J Bacteriol. 1988 Jan;170(1):345–351. doi: 10.1128/jb.170.1.345-351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]