Abstract
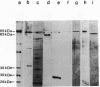
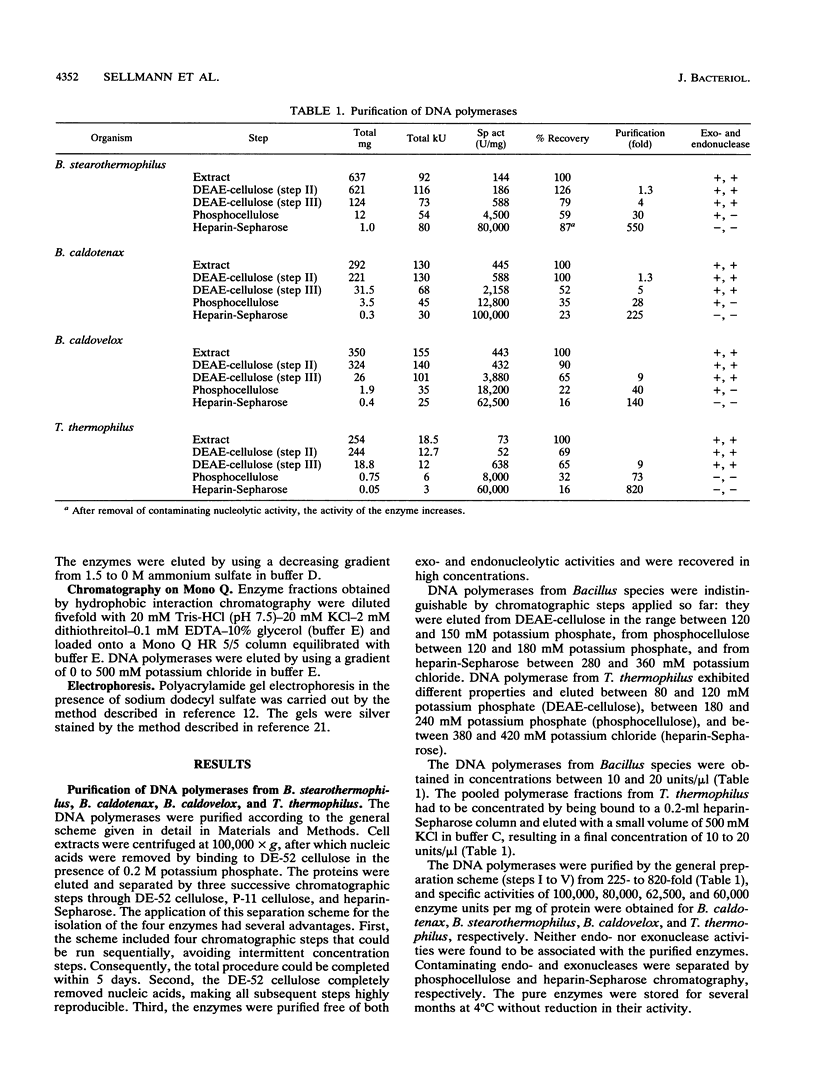
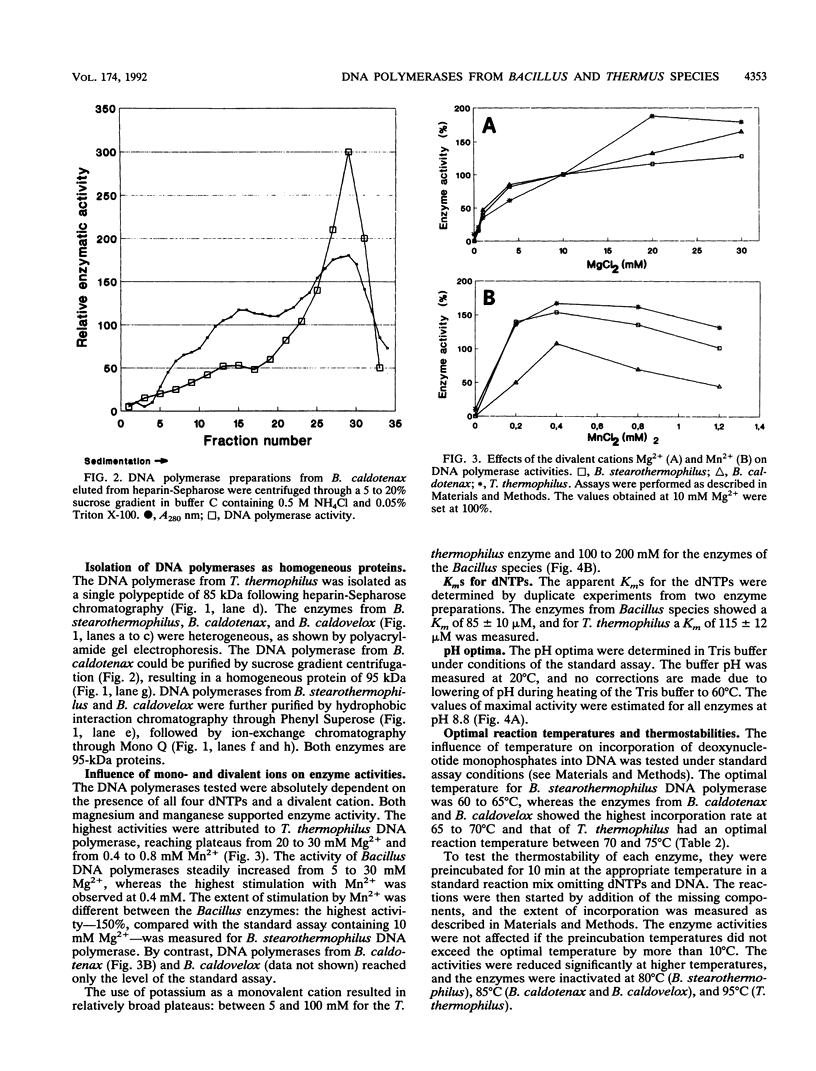
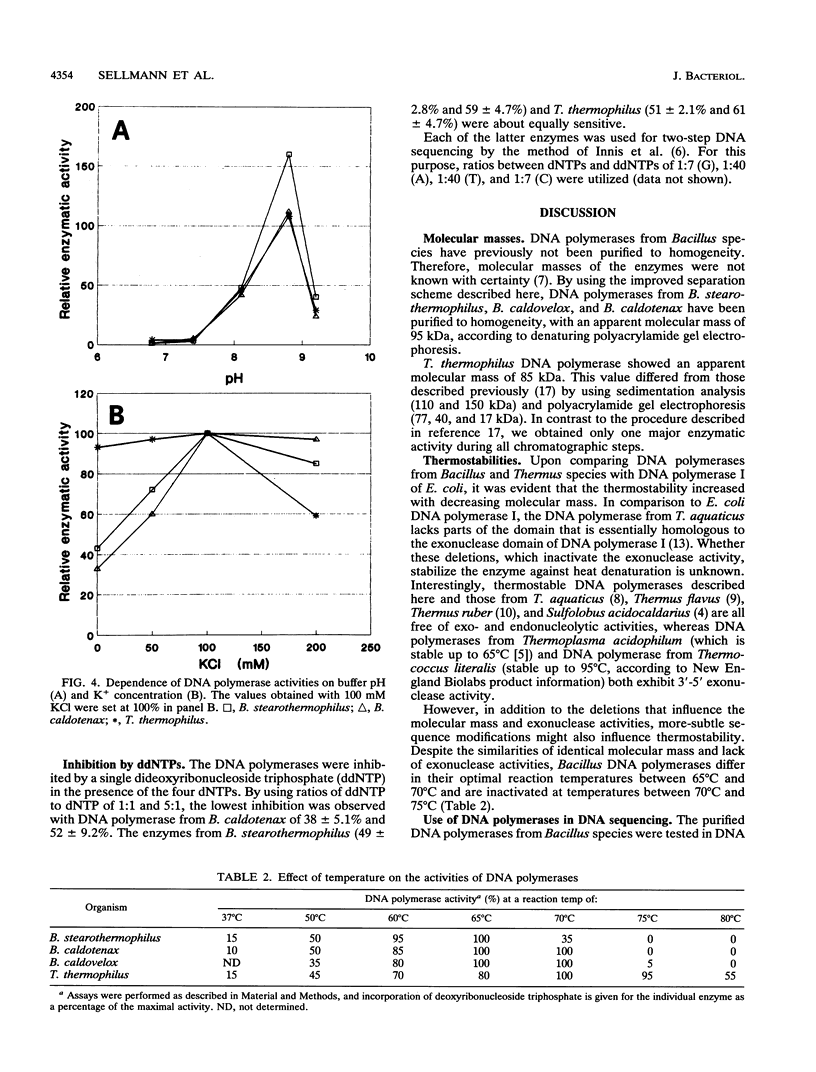
DNA polymerases from Bacillus stearothermophilus, Bacillus caldotenax, and Bacillus caldovelox were purified by chromatography on DEAE-cellulose, phosphocellulose, and heparin-Sepharose and obtained in high yield. The enzyme preparations are free of exo- and endonuclease activities. Additional purification steps, e.g., hydrophobic interaction chromatography and chromatography on a Mono Q column or sucrose density gradient centrifugation, are needed to obtain the enzymes in the form of homogeneous 95-kDa proteins. Each of the three organisms possesses a major DNA polymerase activity comparable to DNA polymerase I. The enzymes require Mg2+ (10 to 30 mM) for optimal activity, although 0.4 mM Mn2+ could substitute for magnesium. The optimal reaction temperatures were lowest in B. stearothermophilus (60 to 65 degrees C) and about equal in B. caldovelox and B. caldotenax (65 to 70 degrees C). The thermal stabilities of the enzymes increased in the same order. The DNA polymerase from Thermus thermophilus was isolated for comparison by using a similar procedure. The enzyme was obtained as a homogeneous 85-kDa protein that was also free of exo- and endonucleolytic activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernad A., Blanco L., Lázaro J. M., Martín G., Salas M. A conserved 3'----5' exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell. 1989 Oct 6;59(1):219–228. doi: 10.1016/0092-8674(89)90883-0. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T. R., Green T. J. A sensitive urea-silver stain method for detecting trace quantities of separated proteins in polyacrylamide gels. Prep Biochem. 1987;17(1):93–99. doi: 10.1080/00327488708062478. [DOI] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elie C., De Recondo A. M., Forterre P. Thermostable DNA polymerase from the archaebacterium Sulfolobus acidocaldarius. Purification, characterization and immunological properties. Eur J Biochem. 1989 Jan 2;178(3):619–626. doi: 10.1111/j.1432-1033.1989.tb14490.x. [DOI] [PubMed] [Google Scholar]
- Hamal A., Forterre P., Elie C. Purification and characterization of a DNA polymerase from the archaebacterium Thermoplasma acidophilum. Eur J Biochem. 1990 Jul 5;190(3):517–521. doi: 10.1111/j.1432-1033.1990.tb15604.x. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboev O. K., Luchkina L. A., Akhmedov A. T., Bekker M. L. Purification and properties of deoxyribonucleic acid polymerase from Bacillus stearothermophilus. J Bacteriol. 1981 Jan;145(1):21–26. doi: 10.1128/jb.145.1.21-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaledin A. S., Sliusarenko A. G., Gorodetskii S. I. Vydelenie i svoistva DNK-polimerazy is ekstremal'no-termofil'noi bakterii Thermus Aquaticus YT1. Biokhimiia. 1980 Apr;45(4):644–651. [PubMed] [Google Scholar]
- Kaledin A. S., Sliusarenko A. G., Gorodetskii S. I. Vydelenie i svoistva DNK-polimerazy iz ekstremal'no-termofil'noi bakterii Thermus ruber. Biokhimiia. 1982 Nov;47(11):1785–1791. [PubMed] [Google Scholar]
- Kaledin A. S., Sliusarenko A. G., Gorodetskii S. I. Vydelenie i svoistva DNK-polimerazy iz ékstremal'no-termofil'noi bakterii Thermus flavus. Biokhimiia. 1981 Sep;46(9):1576–1584. [PubMed] [Google Scholar]
- Kitayama S., Ishizaka Y., Miyai S., Matsuyama A. An exonuclease activity associated with DNA polymerase I of Micrococcus radiodurans. Biochim Biophys Acta. 1978 Aug 23;520(1):122–130. doi: 10.1016/0005-2787(78)90013-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Rüttimann C., Cotorás M., Zaldívar J., Vicuña R. DNA polymerases from the extremely thermophilic bacterium Thermus thermophilus HB-8. Eur J Biochem. 1985 May 15;149(1):41–46. doi: 10.1111/j.1432-1033.1985.tb08890.x. [DOI] [PubMed] [Google Scholar]