Abstract
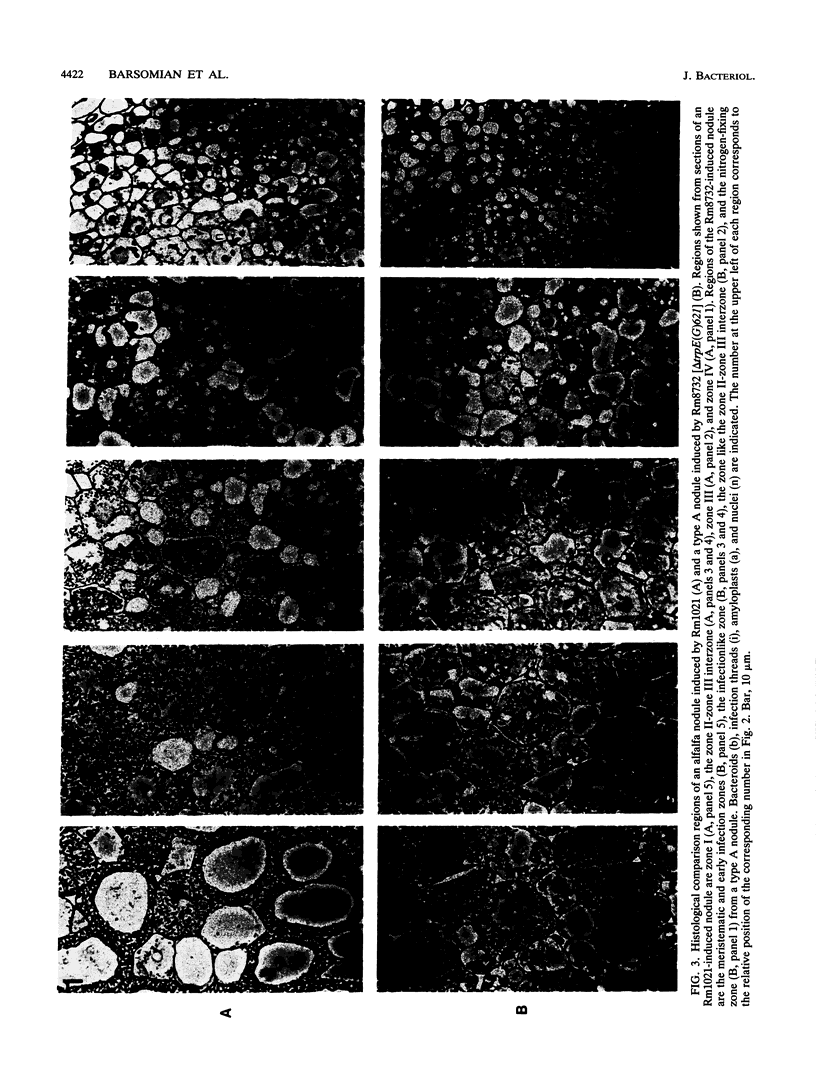
Analyses of Rhizobium meliloti trp auxotrophs suggest that anthranilate biosynthesis by the R. meliloti trpE(G) gene product is necessary during nodule development for establishment of an effective symbiosis. trpE(G) mutants, as well as mutants blocked earlier along this pathway in aromatic amino acid biosynthesis, form nodules on alfalfa that have novel defects. In contrast, R. meliloti trp mutants blocked later in the tryptophan-biosynthetic pathway form normal, pink, nitrogen-fixing nodules. trpE(G) mutants form two types of elongated, defective nodules containing unusually extended invasion zones on alfalfa. One type contains bacteroids in its base and is capable of nitrogen fixation, while the other lacks bacteroids and cannot fix nitrogen. The trpE(G) gene is expressed in normal nodules. Models are discussed to account for these observations, including one in which anthranilate is postulated to act as an in planta siderophore.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae Y. M., Holmgren E., Crawford I. P. Rhizobium meliloti anthranilate synthase gene: cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3471–3478. doi: 10.1128/jb.171.6.3471-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Johnston A. W. Nodulation of legumes by Rhizobium: the recognized root? Cell. 1986 Oct 24;47(2):153–154. doi: 10.1016/0092-8674(86)90436-8. [DOI] [PubMed] [Google Scholar]
- Earl C. D., Ronson C. W., Ausubel F. M. Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J Bacteriol. 1987 Mar;169(3):1127–1136. doi: 10.1128/jb.169.3.1127-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Kunkel B., De Vos G. F., Signer E. R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986 Jul;167(1):66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Glazebrook J., Walker G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989 Feb 24;56(4):661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- Hirsch A. M., Bang M., Ausubel F. M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Bibb M. J., Beringer J. E. Tryptophan genes in Rhizobium--their organization and their transfer to other bacterial genera. Mol Gen Genet. 1978 Oct 24;165(3):323–330. doi: 10.1007/BF00332533. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium genetics. Annu Rev Genet. 1989;23:483–506. doi: 10.1146/annurev.ge.23.120189.002411. [DOI] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Rioux C. R., Jordan D. C., Rattray J. B. Anthranilate-promoted iron uptake in Rhizobium leguminosarum. Arch Biochem Biophys. 1986 Jul;248(1):183–189. doi: 10.1016/0003-9861(86)90415-7. [DOI] [PubMed] [Google Scholar]
- Rioux C. R., Jordan D. C., Rattray J. B. Iron requirement of Rhizobium leguminosarum and secretion of anthranilic acid during growth on an iron-deficient medium. Arch Biochem Biophys. 1986 Jul;248(1):175–182. doi: 10.1016/0003-9861(86)90414-5. [DOI] [PubMed] [Google Scholar]
- Sharma S. B., Signer E. R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 1990 Mar;4(3):344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J., de Billy F., Camut S., Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990 Aug;172(8):4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]