Abstract
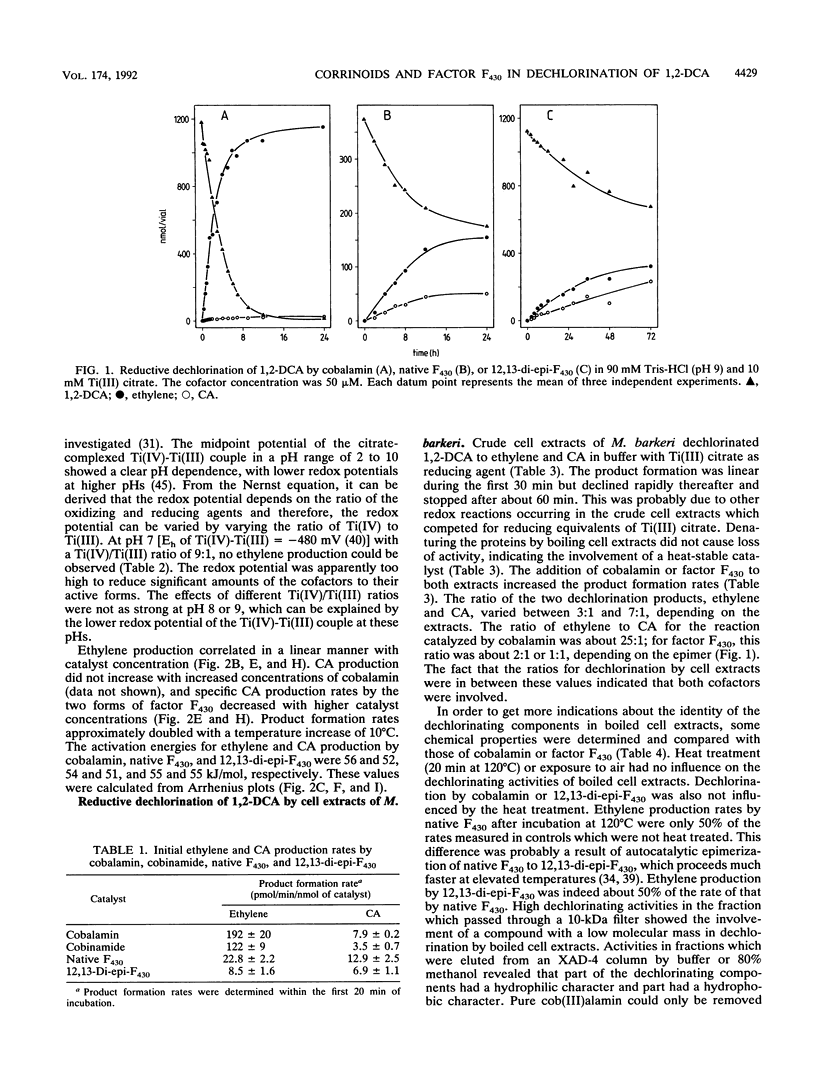
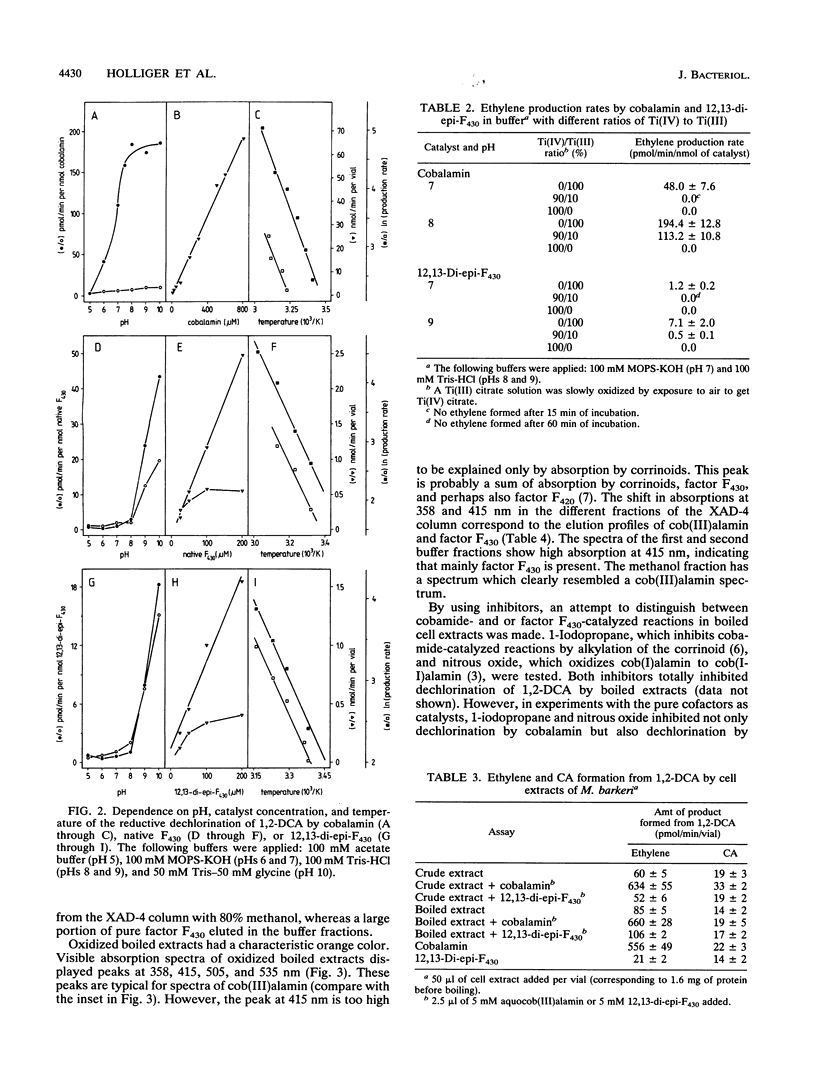
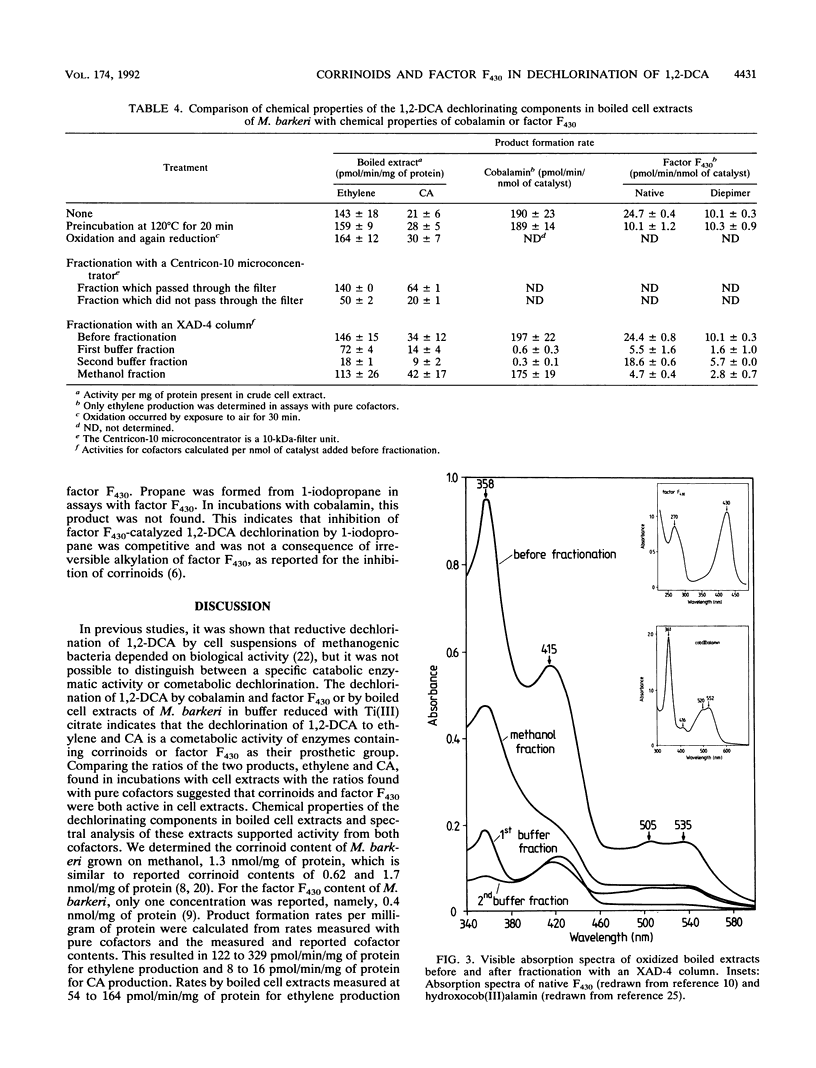
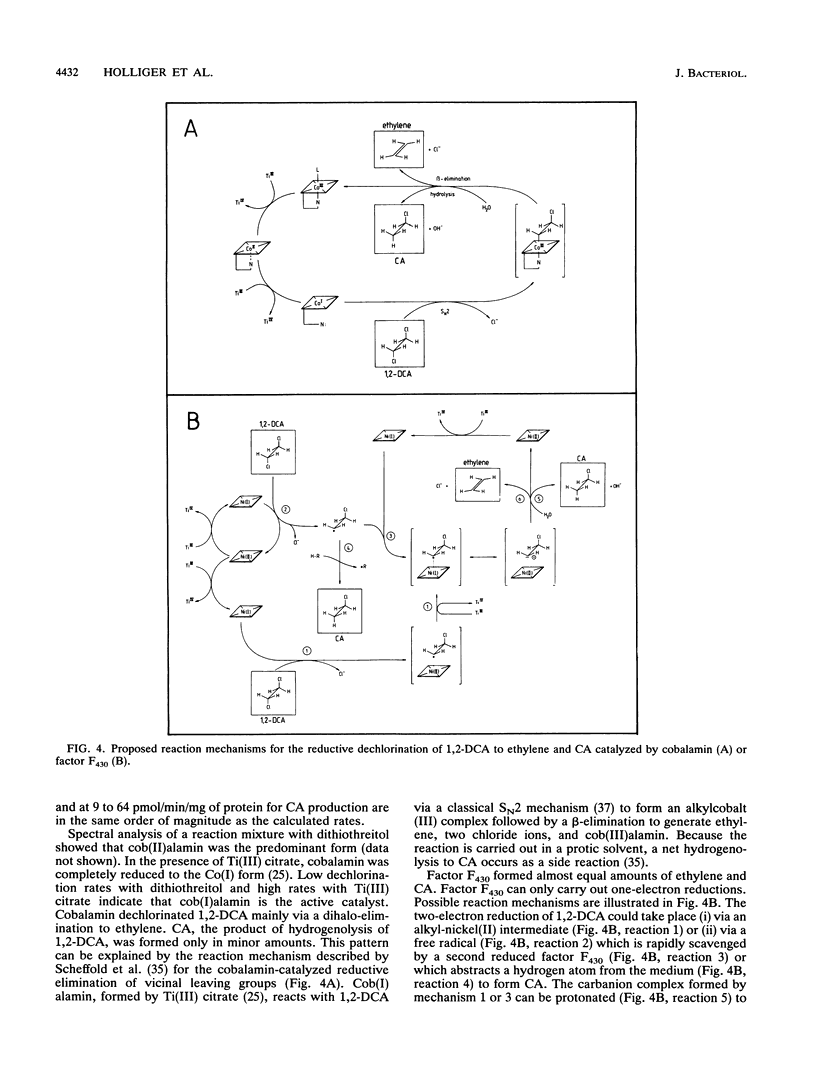
Cobalamin and the native and diepimeric forms of factor F430 catalyzed the reductive dechlorination of 1,2-dichloroethane (1,2-DCA) to ethylene or chloroethane (CA) in a buffer with Ti(III) citrate as the electron donor. Ethylene was the major product in the cobalamin-catalyzed transformation, and the ratio of ethylene to CA formed was 25:1. Native F430 and 12,13-di-epi-F430 produced ethylene and CA in ratios of about 2:1 and 1:1, respectively. Cobalamin dechlorinated 1,2-DCA much faster than did factor F430. Dechlorination rates by all three catalysts showed a distinct pH dependence, correlated in a linear manner with the catalyst concentration and doubled with a temperature increase of 10 degrees C. Crude and boiled cell extracts of Methanosarcina barkeri also dechlorinated 1,2-DCA to ethylene and CA with Ti(III) citrate as the reductant. The catalytic components in boiled extracts were heat and oxygen stable and had low molecular masses. Fractionation of boiled extracts by a hydrophobic interaction column revealed that part of the dechlorinating components had a hydrophilic and part had a hydrophobic character. These chemical properties of the dechlorinating components and spectral analysis of boiled extracts indicated that corrinoids or factor F430 was responsible for the dechlorinations. The ratios of 3:1 to 7:1 of ethylene and CA formed by cell extracts suggested that both cofactors were concomitantly active.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROT N., WEISSBACH H. ENZYMATIC SYNTHESIS OF METHIONINE. CHEMICAL ALKYLATION OF THE ENZYME-BOUND COBAMIDE. J Biol Chem. 1965 Jul;240:3064–3070. [PubMed] [Google Scholar]
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Biocca S., Nasi S., Calissano P. Hidden receptors for nerve growth factor in PC12 cells. Eur J Biochem. 1983 Sep 15;135(2):285–290. doi: 10.1111/j.1432-1033.1983.tb07650.x. [DOI] [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G., Konheiser U., Piechulla K., Thauer R. K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981 Nov;148(2):459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli C., Tschan T., Scholtz R., Cook A. M., Leisinger T. Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl Environ Microbiol. 1988 Nov;54(11):2819–2824. doi: 10.1128/aem.54.11.2819-2824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Fathepure B. Z., Boyd S. A. Dependence of tetrachloroethylene dechlorination on methanogenic substrate consumption by Methanosarcina sp. strain DCM. Appl Environ Microbiol. 1988 Dec;54(12):2976–2980. doi: 10.1128/aem.54.12.2976-2980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure B. Z., Nengu J. P., Boyd S. A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987 Nov;53(11):2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälli R., McCarty P. L. Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridium sp. Appl Environ Microbiol. 1989 Apr;55(4):837–844. doi: 10.1128/aem.55.4.837-844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stams A. J., Zehnder A. J. Reductive dechlorination of 1,2-dichloroethane and chloroethane by cell suspensions of methanogenic bacteria. Biodegradation. 1990;1(4):253–261. doi: 10.1007/BF00119762. [DOI] [PubMed] [Google Scholar]
- Kengen S. W., Daas P. J., Duits E. F., Keltjens J. T., van der Drift C., Vogels G. D. Isolation of a 5-hydroxybenzimidazolyl cobamide-containing enzyme involved in the methyltetrahydromethanopterin: coenzyme M methyltransferase reaction in Methanobacterium thermoautotrophicum. Biochim Biophys Acta. 1992 Feb 1;1118(3):249–260. doi: 10.1016/0167-4838(92)90282-i. [DOI] [PubMed] [Google Scholar]
- Krone U. E., Thauer R. K., Hogenkamp H. P., Steinbach K. Reductive formation of carbon monoxide from CCl4 and FREONs 11, 12, and 13 catalyzed by corrinoids. Biochemistry. 1991 Mar 12;30(10):2713–2719. doi: 10.1021/bi00224a020. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Mikesell M. D., Boyd S. A. Dechlorination of chloroform by methanosarcina strains. Appl Environ Microbiol. 1990 Apr;56(4):1198–1201. doi: 10.1128/aem.56.4.1198-1201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauzer G. N., Deutsch E. Reactions of cobalt(I) supernucleophiles. The alkylation of vitamin B12s cobaloximes(I), and related compounds. J Am Chem Soc. 1969 Jun 4;91(12):3341–3350. doi: 10.1021/ja01040a041. [DOI] [PubMed] [Google Scholar]
- Shiemke A. K., Hamilton C. L., Scott R. A. Structural heterogeneity and purification of protein-free F430 from the cytoplasm of Methanobacterium thermoautotrophicum. J Biol Chem. 1988 Apr 25;263(12):5611–5616. [PubMed] [Google Scholar]
- Shiemke A. K., Shelnutt J. A., Scott R. A. Coordination chemistry of F430. Axial ligation equilibrium between square-planar and bis-aquo species in aqueous solution. J Biol Chem. 1989 Jul 5;264(19):11236–11245. [PubMed] [Google Scholar]
- Stupperich E., Steiner I., Rühlemann M. Isolation and analysis of bacterial cobamides by high-performance liquid chromatography. Anal Biochem. 1986 Jun;155(2):365–370. doi: 10.1016/0003-2697(86)90447-1. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Möller-Zinkhan D., Spormann A. M. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol. 1989;43:43–67. doi: 10.1146/annurev.mi.43.100189.000355. [DOI] [PubMed] [Google Scholar]
- Wade R. S., Castro C. E. Oxidation of iron (II) porphyrins by alkyl halides. J Am Chem Soc. 1973 Jan 10;95(1):226–230. doi: 10.1021/ja00782a040. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]



