Abstract
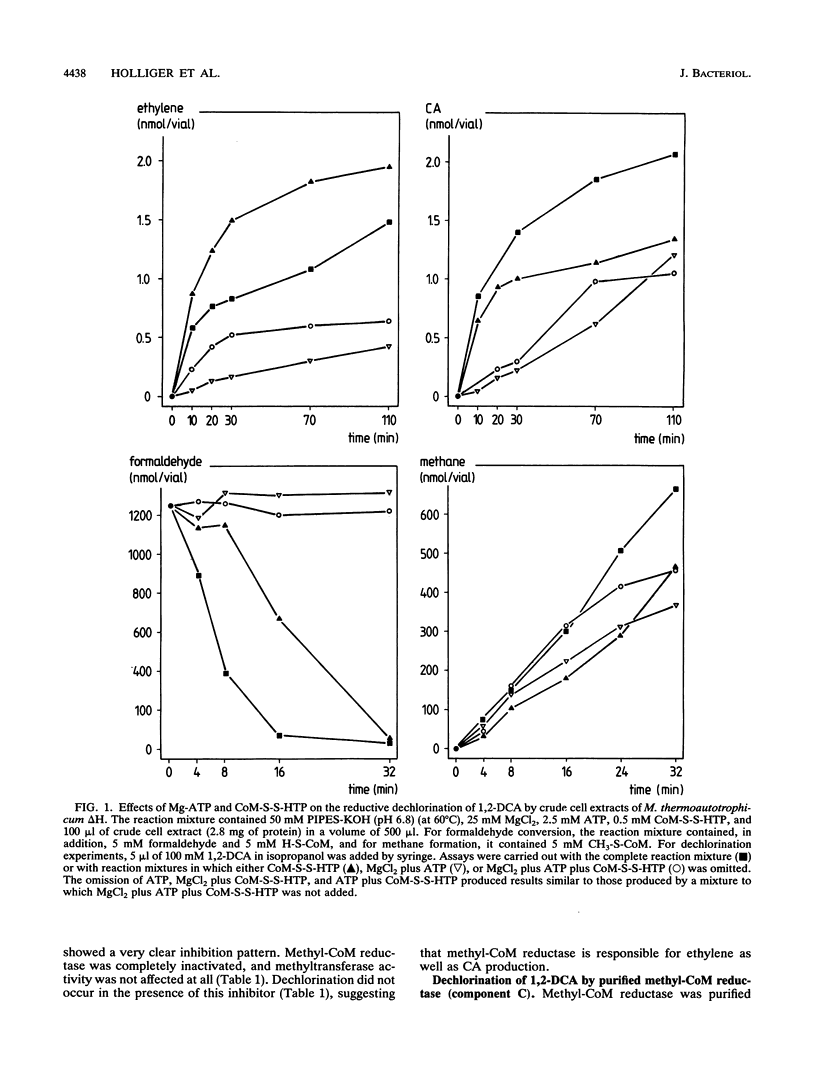
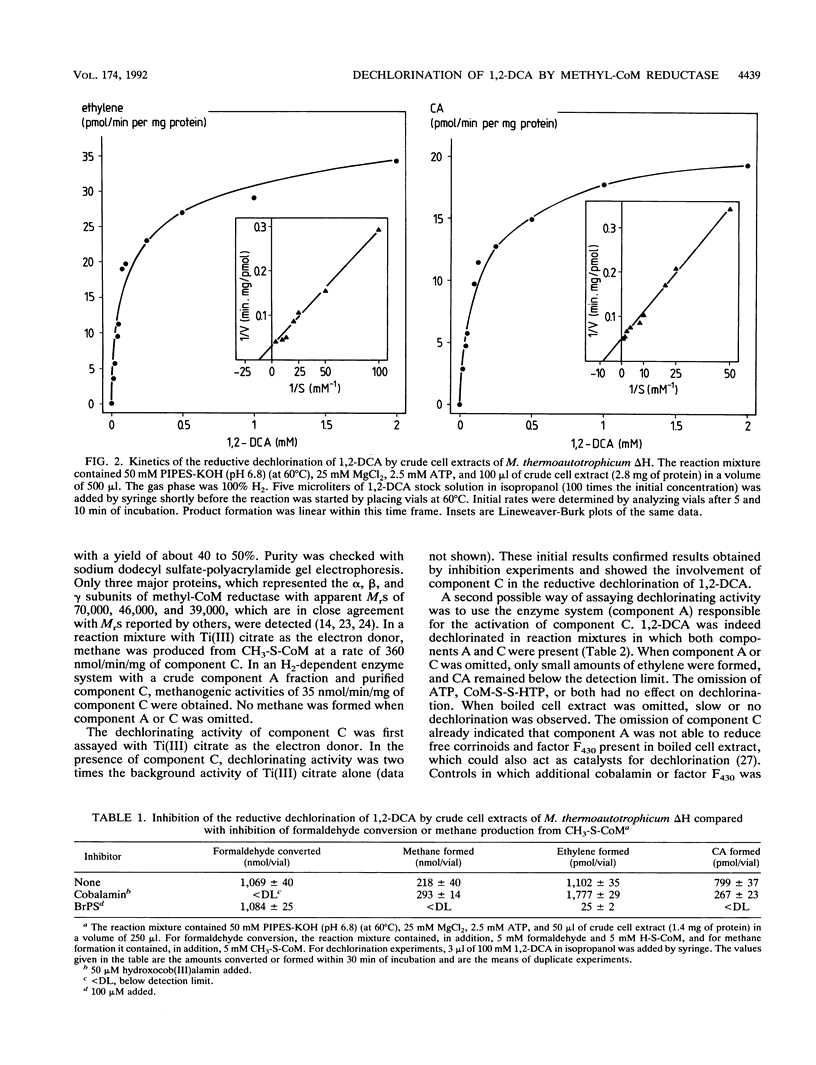
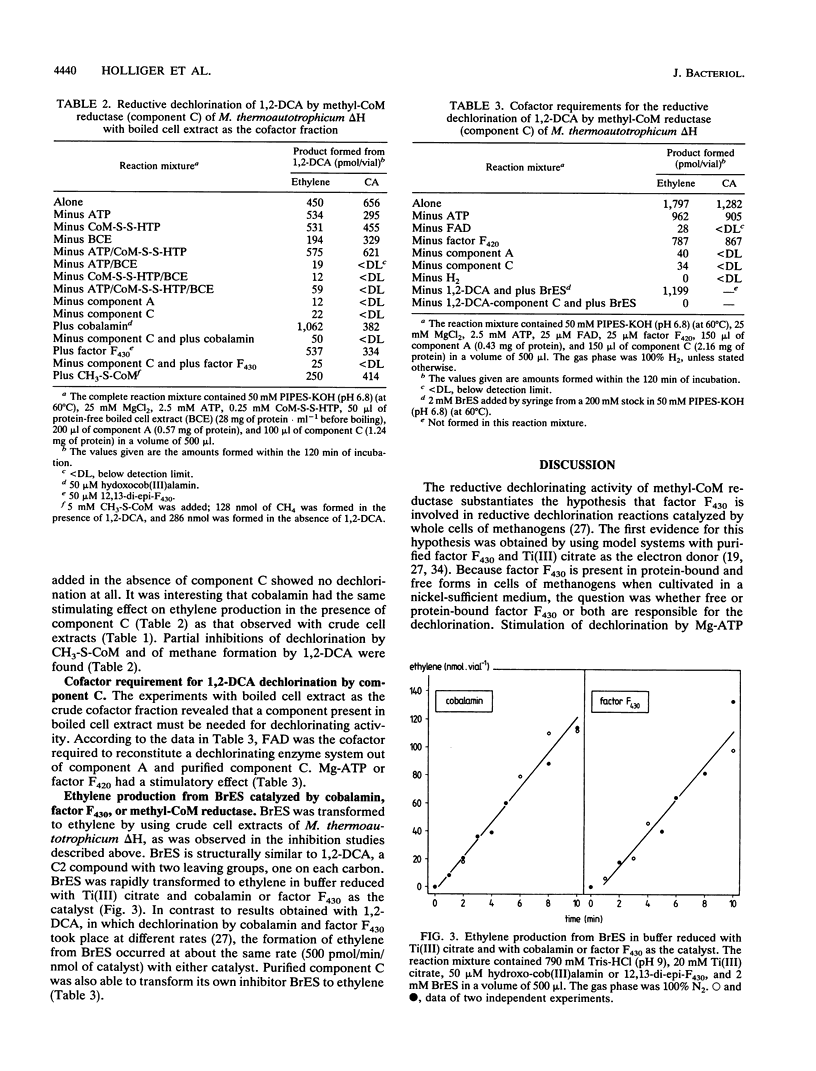
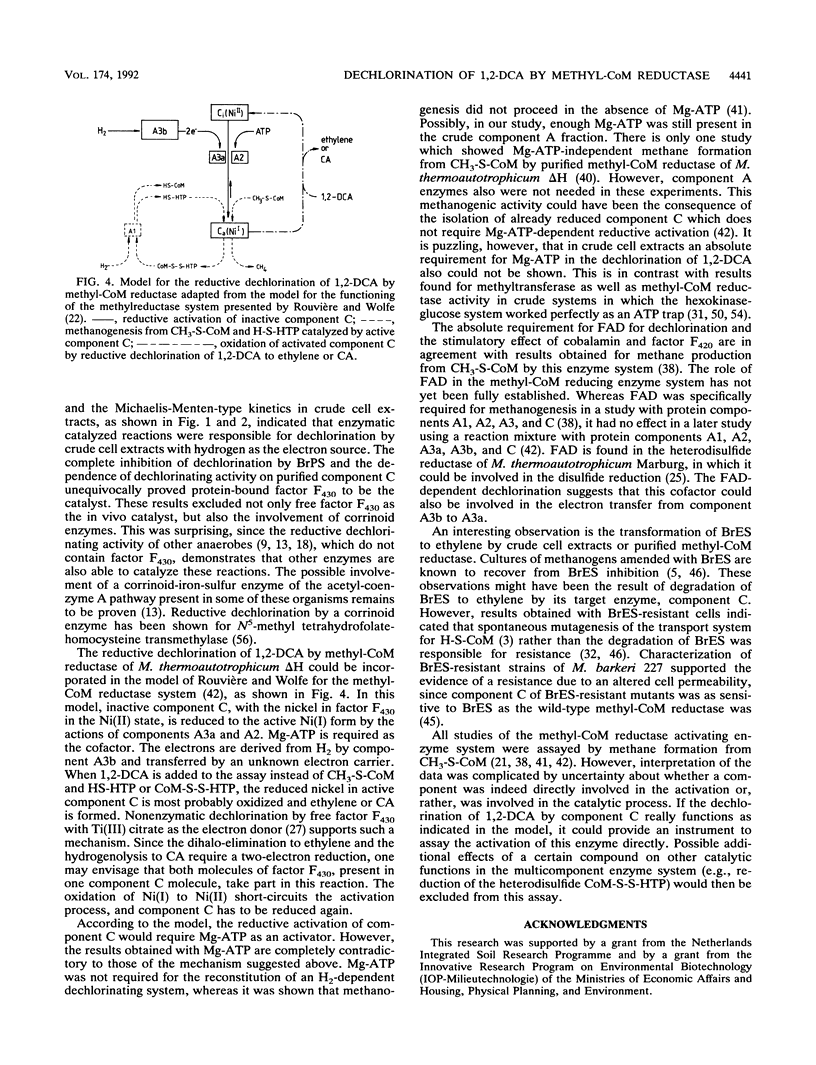
Reductive dechlorination of 1,2-dichloroethane (1,2-DCA) to ethylene and chloroethane (CA) by crude cell extracts of Methanobacterium thermoautotrophicum delta H with H2 as the electron donor was stimulated by Mg-ATP. The heterodisulfide of coenzyme M (CoM) and 7-mercaptoheptanoylthreonine phosphate together with Mg-ATP partially inhibited ethylene production but stimulated CA production compared Mg-ATP alone. The pH optimum for the dechlorination was 6.8 (at 60 degrees C). Michaelis-Menten kinetics for initial product formation rates with different 1,2-DCA concentrations indicated the enzymatic character of the dechlorination. Apparent Kms for 1,2-DCA of 89 and 119 microM and Vmaxs of 34 and 20 pmol/min/mg of protein were estimated for ethylene and CA production, respectively. 3-Bromopropanesulfonate, a specific inhibitor for methyl-CoM reductase, completely inhibited dechlorination of 1,2-DCA. Purified methyl-CoM reductase, together with flavin adenine dinucleotide and a crude component A fraction which reduced the nickel of factor F430 in methyl-CoM reductase, converted 1,2-DCA to ethylene and CA with H2 as the electron donor. In this system, methyl-CoM reductase was also able to transform its own inhibitor 2-bromoethanesulfonate to ethylene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. J Bacteriol. 1979 Jan;137(1):264–273. doi: 10.1128/jb.137.1.264-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. V., Harder S. R., Ragsdale S. W., Matthews R. G. Mechanism of reductive activation of cobalamin-dependent methionine synthase: an electron paramagnetic resonance spectroelectrochemical study. Biochemistry. 1990 Feb 6;29(5):1129–1135. doi: 10.1021/bi00457a005. [DOI] [PubMed] [Google Scholar]
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik T. A., Olson K. D., Noll K. M., Wolfe R. S. Evidence that the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate is a product of the methylreductase reaction in Methanobacterium. Biochem Biophys Res Commun. 1987 Dec 16;149(2):455–460. doi: 10.1016/0006-291x(87)90389-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao X. J., Krzycki J. A. Acetate-dependent methylation of two corrinoid proteins in extracts of Methanosarcina barkeri. J Bacteriol. 1991 Sep;173(17):5439–5448. doi: 10.1128/jb.173.17.5439-5448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle C. S., DeWitt J. T., McCarty P. L. Reductive dehalogenation of carbon tetrachloride by Escherichia coli K-12. Appl Environ Microbiol. 1990 Nov;56(11):3247–3254. doi: 10.1128/aem.56.11.3247-3254.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G., Konheiser U., Piechulla K., Thauer R. K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981 Nov;148(2):459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli C., Tschan T., Scholtz R., Cook A. M., Leisinger T. Transformation of tetrachloromethane to dichloromethane and carbon dioxide by Acetobacterium woodii. Appl Environ Microbiol. 1988 Nov;54(11):2819–2824. doi: 10.1128/aem.54.11.2819-2824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellermann J., Hedderich R., Böcher R., Thauer R. K. The final step in methane formation. Investigations with highly purified methyl-CoM reductase (component C) from Methanobacterium thermoautotrophicum (strain Marburg). Eur J Biochem. 1988 Mar 15;172(3):669–677. doi: 10.1111/j.1432-1033.1988.tb13941.x. [DOI] [PubMed] [Google Scholar]
- Ellermann J., Rospert S., Thauer R. K., Bokranz M., Klein A., Voges M., Berkessel A. Methyl-coenzyme-M reductase from Methanobacterium thermoautotrophicum (strain Marburg). Purity, activity and novel inhibitors. Eur J Biochem. 1989 Sep 1;184(1):63–68. doi: 10.1111/j.1432-1033.1989.tb14990.x. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Gälli R., McCarty P. L. Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridium sp. Appl Environ Microbiol. 1989 Apr;55(4):837–844. doi: 10.1128/aem.55.4.837-844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderich R., Berkessel A., Thauer R. K. Purification and properties of heterodisulfide reductase from Methanobacterium thermoautotrophicum (strain Marburg). Eur J Biochem. 1990 Oct 5;193(1):255–261. doi: 10.1111/j.1432-1033.1990.tb19331.x. [DOI] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stams A. J., Zehnder A. J. Reductive dechlorination of 1,2-dichloroethane and chloroethane by cell suspensions of methanogenic bacteria. Biodegradation. 1990;1(4):253–261. doi: 10.1007/BF00119762. [DOI] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stupperich E., Stams A. J., Zehnder A. J. Evidence for the involvement of corrinoids and factor F430 in the reductive dechlorination of 1,2-dichloroethane by Methanosarcina barkeri. J Bacteriol. 1992 Jul;174(13):4427–4434. doi: 10.1128/jb.174.13.4427-4434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltjens J. T., van Erp R., Mooijaart R. J., van der Drift C., Vogels G. D. Inorganic pyrophosphate synthesis during methanogenesis from methylcoenzyme M by cell-free extracts of Methanobacterium thermoautotrophicum (strain delta H). Eur J Biochem. 1988 Mar 1;172(2):471–476. doi: 10.1111/j.1432-1033.1988.tb13912.x. [DOI] [PubMed] [Google Scholar]
- Kengen S. W., Daas P. J., Duits E. F., Keltjens J. T., van der Drift C., Vogels G. D. Isolation of a 5-hydroxybenzimidazolyl cobamide-containing enzyme involved in the methyltetrahydromethanopterin: coenzyme M methyltransferase reaction in Methanobacterium thermoautotrophicum. Biochim Biophys Acta. 1992 Feb 1;1118(3):249–260. doi: 10.1016/0167-4838(92)90282-i. [DOI] [PubMed] [Google Scholar]
- Krone U. E., Laufer K., Thauer R. K., Hogenkamp H. P. Coenzyme F430 as a possible catalyst for the reductive dehalogenation of chlorinated C1 hydrocarbons in methanogenic bacteria. Biochemistry. 1989 Dec 26;28(26):10061–10065. doi: 10.1021/bi00452a027. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S., Linder D., Ellermann J., Thauer R. K. Two genetically distinct methyl-coenzyme M reductases in Methanobacterium thermoautotrophicum strain Marburg and delta H. Eur J Biochem. 1990 Dec 27;194(3):871–877. doi: 10.1111/j.1432-1033.1990.tb19481.x. [DOI] [PubMed] [Google Scholar]
- Rouvière P. E., Bobik T. A., Wolfe R. S. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Sep;170(9):3946–3952. doi: 10.1128/jb.170.9.3946-3952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. Component A3 of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H: resolution into two components. J Bacteriol. 1989 Sep;171(9):4556–4562. doi: 10.1128/jb.171.9.4556-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H., Albracht S. P., Coremans J. M., Fuchs G. Purification and some properties of the corrinoid-containing membrane protein from Methanobacterium thermoautotrophicum. Eur J Biochem. 1988 Feb 1;171(3):589–597. doi: 10.1111/j.1432-1033.1988.tb13829.x. [DOI] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Smith M. R. Reversal of 2-bromoethanesulfonate inhibition of methanogenesis in Methanosarcina sp. J Bacteriol. 1983 Nov;156(2):516–523. doi: 10.1128/jb.156.2.516-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Möller-Zinkhan D., Spormann A. M. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol. 1989;43:43–67. doi: 10.1146/annurev.mi.43.100189.000355. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by ATP. J Bacteriol. 1983 May;154(2):640–649. doi: 10.1128/jb.154.2.640-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Wolfe R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry. 1968 May;7(5):1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Pouwels A., Houwen F., van der Drift C., Vogels G. D. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol. 1983 Jun;134(3):238–242. doi: 10.1007/BF00407765. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Sliepenbeek H. T., Houwen F. P., van der Drift C., Vogels G. D. Activation and inactivation of methanol: 2-mercaptoethanesulfonic acid methyltransferase from Methanosarcina barkeri. J Bacteriol. 1983 Jan;153(1):6–11. doi: 10.1128/jb.153.1.6-11.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden P., te Brömmelstroet B. W., Poirot C. M., van der Drift C., Vogels G. D. Purification and properties of methanol:5-hydroxybenzimidazolylcobamide methyltransferase from Methanosarcina barkeri. J Bacteriol. 1984 Nov;160(2):629–635. doi: 10.1128/jb.160.2.629-635.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



