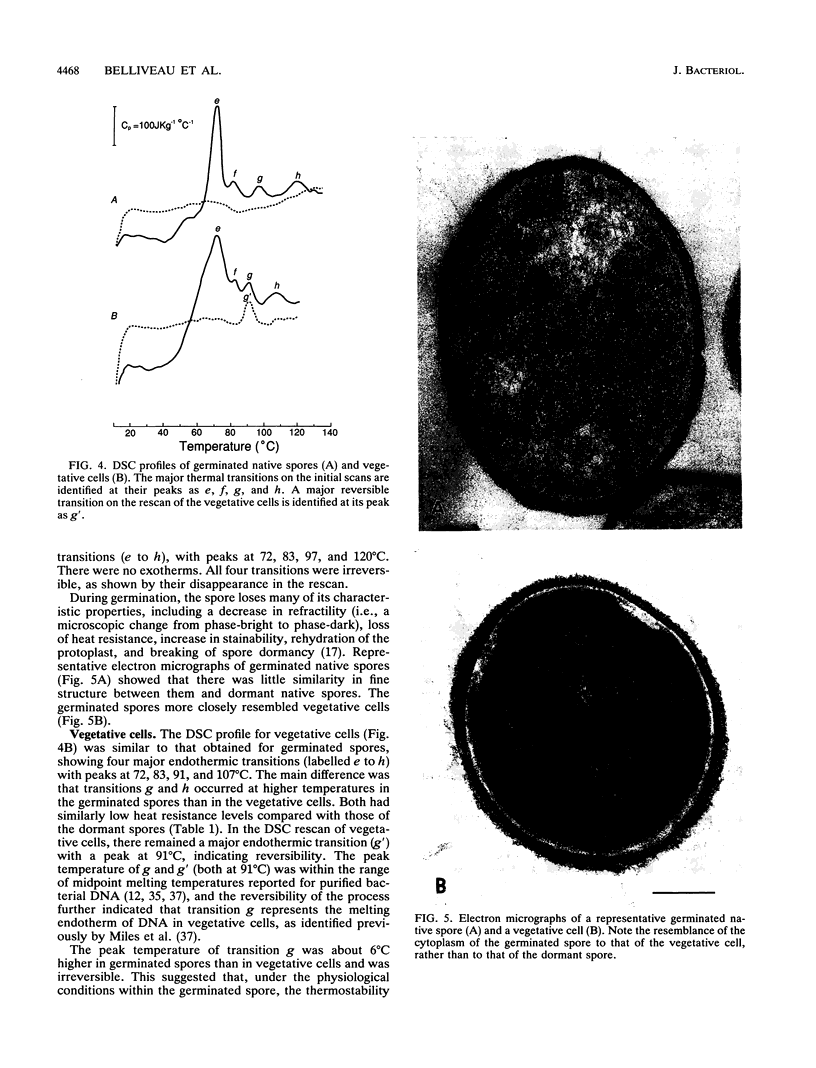
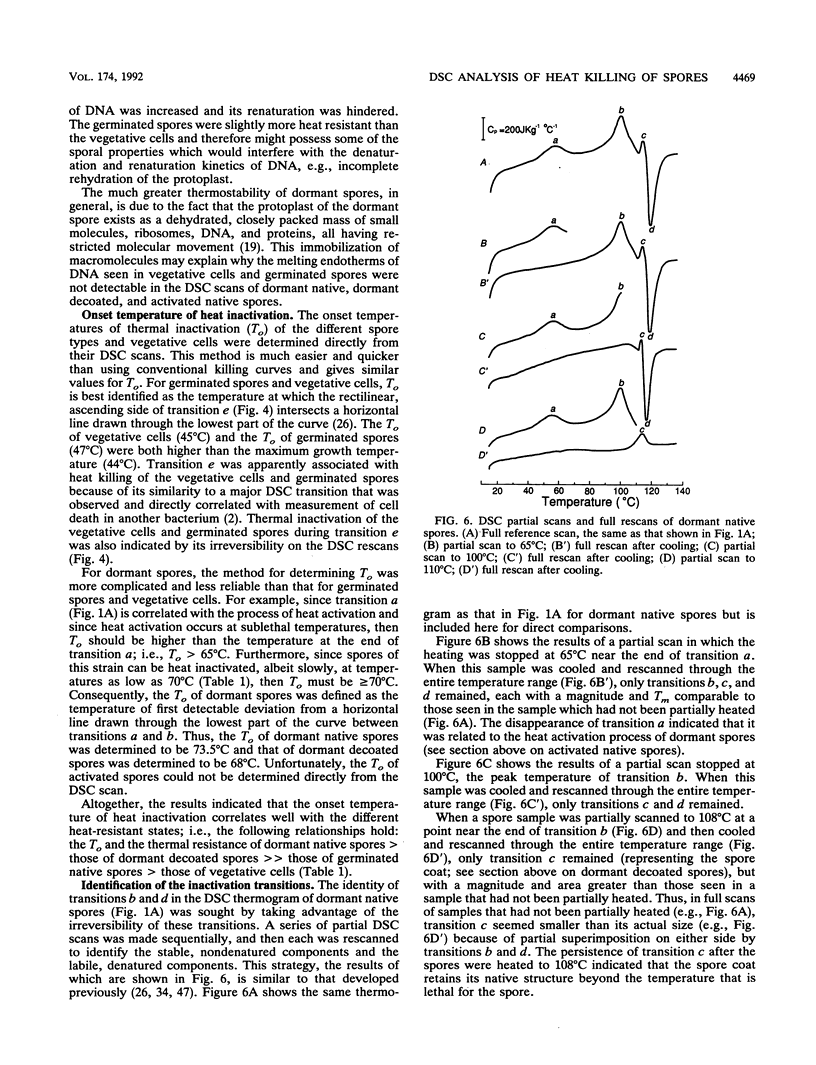
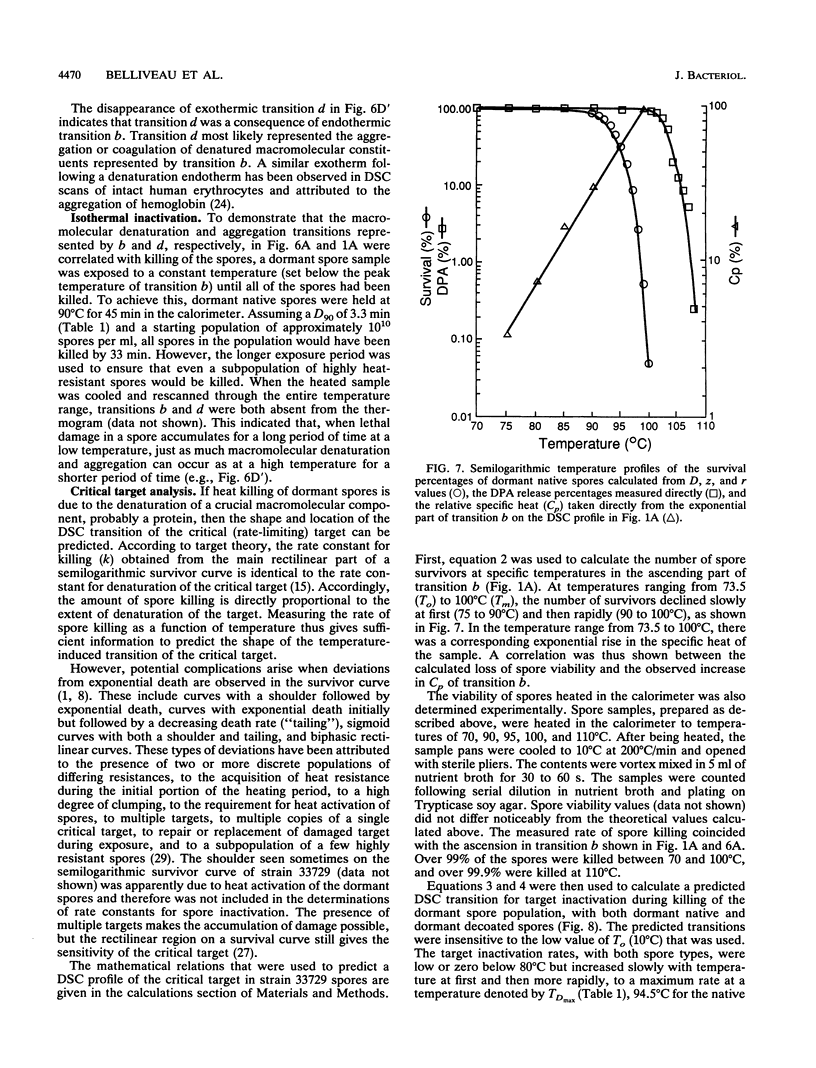
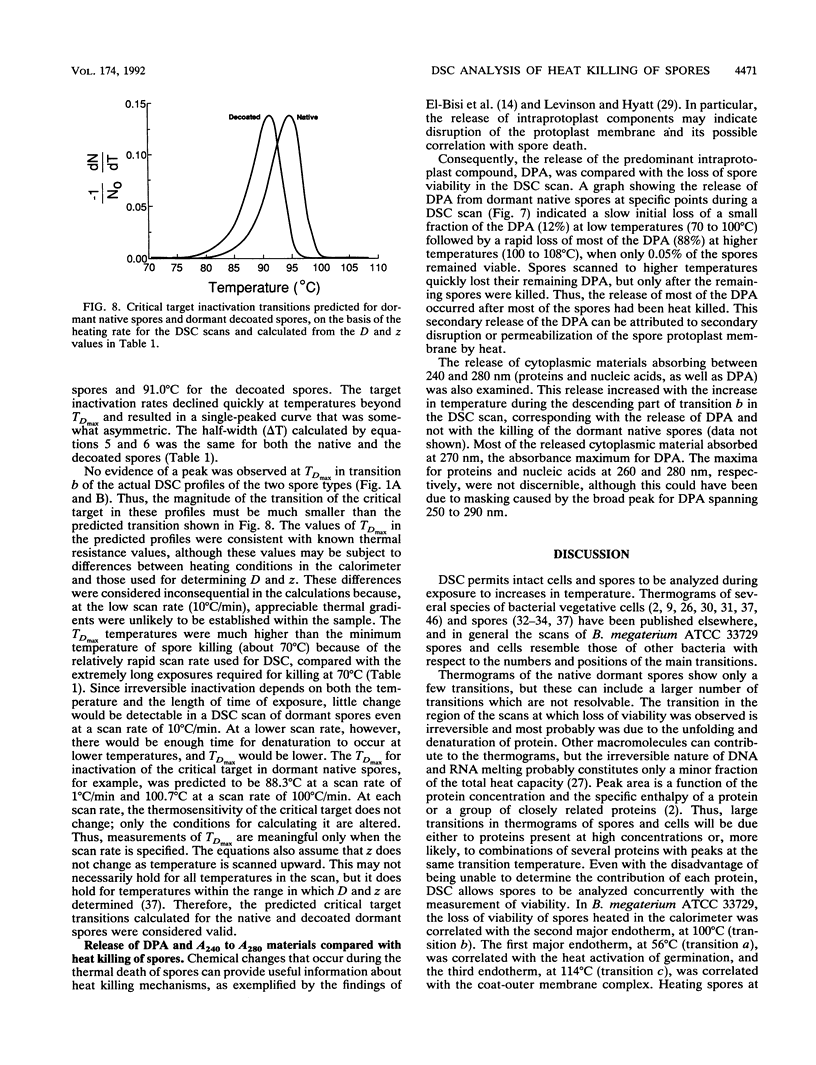
Abstract
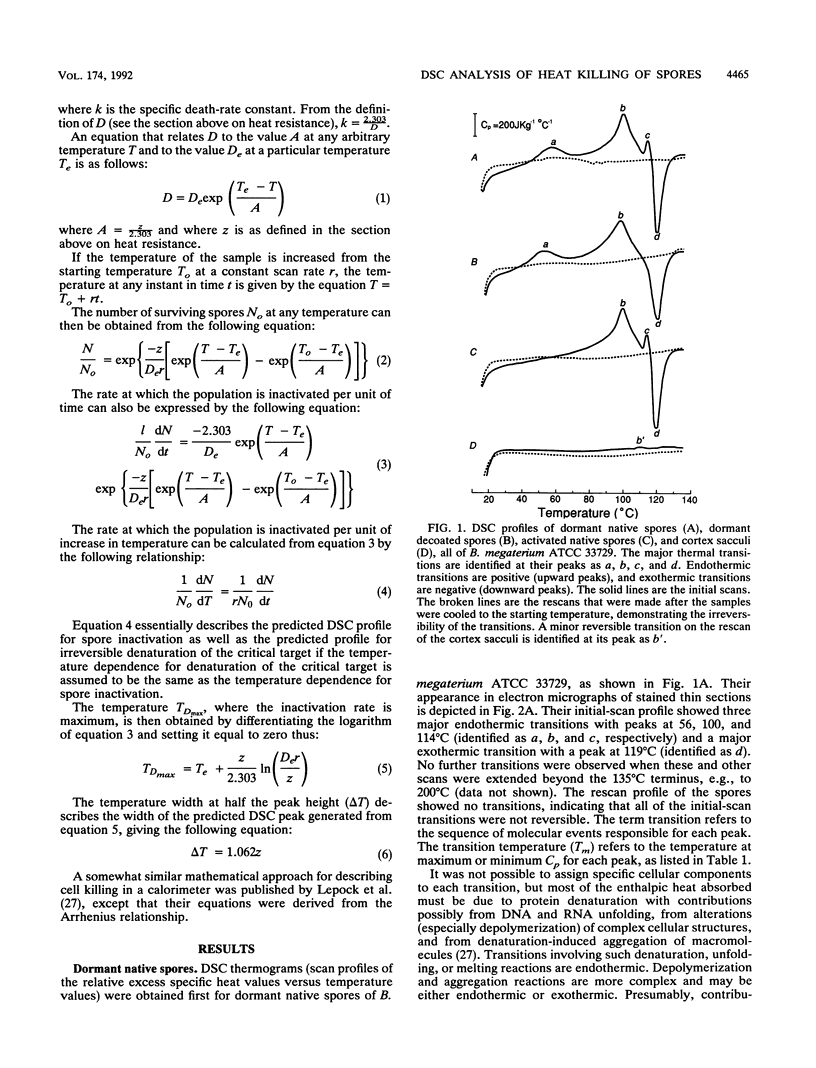
Thermograms of the exosporium-lacking dormant spores of Bacillus megaterium ATCC 33729, obtained by differential scanning calorimetry, showed three major irreversible endothermic transitions with peaks at 56, 100, and 114 degrees C and a major irreversible exothermic transition with a peak at 119 degrees C. The 114 degrees C transition was identified with coat proteins, and the 56 degrees C transition was identified with heat inactivation. Thermograms of the germinated spores and vegetative cells were much alike, including an endothermic transition attributable to DNA. The ascending part of the main endothermic 100 degrees C transition in the dormant-spore thermograms corresponded to a first-order reaction and was correlated with spore death; i.e., greater than 99.9% of the spores were killed when the transition peak was reached. The maximum death rate of the dormant spores during calorimetry, calculated from separately measured D and z values, occurred at temperatures above the 73 degrees C onset of thermal denaturation and was equivalent to the maximum inactivation rate calculated for the critical target. Most of the spore killing occurred before the release of most of the dipicolinic acid and other intraprotoplast materials. The exothermic 119 degrees C transition was a consequence of the endothermic 100 degrees C transition and probably represented the aggregation of intraprotoplast spore components. Taken together with prior evidence, the results suggest that a crucial protein is the rate-limiting primary target in the heat killing of dormant bacterial spores.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ababouch L., Dikra A., Busta F. F. Tailing of survivor curves of clostridial spores heated in edible oils. J Appl Bacteriol. 1987 Jun;62(6):503–511. doi: 10.1111/j.1365-2672.1987.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Anderson W. A., Hedges N. D., Jones M. V., Cole M. B. Thermal inactivation of Listeria monocytogenes studied by differential scanning calorimetry. J Gen Microbiol. 1991 Jun;137(6):1419–1424. doi: 10.1099/00221287-137-6-1419. [DOI] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. III. Permeation relative to germination. J Bacteriol. 1962 Feb;83:301–308. doi: 10.1128/jb.83.2.301-308.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Heat shock affects permeability and resistance of Bacillus stearothermophilus spores. Appl Environ Microbiol. 1988 Oct;54(10):2515–2520. doi: 10.1128/aem.54.10.2515-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau B. H., Beaman T. C., Gerhardt P. Heat Resistance Correlated with DNA Content in Bacillus megaterium Spores. Appl Environ Microbiol. 1990 Sep;56(9):2919–2921. doi: 10.1128/aem.56.9.2919-2921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf O. Tailing of survival curves of bacterial spores. J Appl Bacteriol. 1977 Feb;42(1):1–19. doi: 10.1111/j.1365-2672.1977.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Cho K. C., Chow K. C., Mark K. K. A calorimetric study of the thermal transitions of Halobacterium cutirubrum. Can J Biochem. 1982 Apr;60(4):419–421. doi: 10.1139/o82-049. [DOI] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., EVANCHIK Z., HALVORSON H. O., HASTINGS J. W. ACTIVATION OF BACTERIAL ENDOSPORES. J Bacteriol. 1964 Aug;88:313–318. doi: 10.1128/jb.88.2.313-318.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa T., Beaman T. C., Pankratz H. S., Nakashio S., Corner T. R., Gerhardt P. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984 Aug;159(2):624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Bayne H., Markus J. Relationship of hyperthermia-induced hemolysis of human erythrocytes to the thermal denaturation of membrane proteins. Biochim Biophys Acta. 1989 Apr 14;980(2):191–201. doi: 10.1016/0005-2736(89)90399-4. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Heynen M. P., Nishio J., Waters B., Ritchie K. P., Kruuv J. Increased thermostability of thermotolerant CHL V79 cells as determined by differential scanning calorimetry. J Cell Physiol. 1990 Mar;142(3):628–634. doi: 10.1002/jcp.1041420324. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Inniss W. E. Thermal analysis of bacteria by differential scanning calorimetry: relationship of protein denaturation in situ to maximum growth temperature. Biochim Biophys Acta. 1990 Oct 15;1055(1):19–26. doi: 10.1016/0167-4889(90)90086-s. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Rodahl A. M., Kruuv J. Thermal analysis of CHL V79 cells using differential scanning calorimetry: implications for hyperthermic cell killing and the heat shock response. J Cell Physiol. 1988 Oct;137(1):14–24. doi: 10.1002/jcp.1041370103. [DOI] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Distribution and correlation of events during thermal inactivation of Bacillus megaterium spores. J Bacteriol. 1971 Oct;108(1):111–121. doi: 10.1128/jb.108.1.111-121.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Heat activation kinetics of Bacillus megaterium spores. Biochem Biophys Res Commun. 1969 Dec 4;37(6):909–916. doi: 10.1016/0006-291x(69)90217-4. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mackey B. M., Miles C. A., Parsons S. E., Seymour D. A. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J Gen Microbiol. 1991 Oct;137(10):2361–2374. doi: 10.1099/00221287-137-10-2361. [DOI] [PubMed] [Google Scholar]
- Mackey B. M., Parsons S. E., Miles C. A., Owen R. J. The relationship between the base composition of bacterial DNA and its intracellular melting temperature as determined by differential scanning calorimetry. J Gen Microbiol. 1988 May;134(5):1185–1195. doi: 10.1099/00221287-134-5-1185. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Kagami I., Koga S. Thermal analysis of the spores of Bacillus cereus with special reference to heat activation. Can J Microbiol. 1978 Nov;24(11):1331–1334. doi: 10.1139/m78-214. [DOI] [PubMed] [Google Scholar]
- Miles C. A., Mackey B. M., Parsons S. E. Differential scanning calorimetry of bacteria. J Gen Microbiol. 1986 Apr;132(4):939–952. doi: 10.1099/00221287-132-4-939. [DOI] [PubMed] [Google Scholar]
- Moats W. A. Kinetics of thermal death of bacteria. J Bacteriol. 1971 Jan;105(1):165–171. doi: 10.1128/jb.105.1.165-171.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly B. J., Shafa F., Gerhardt P. Structural details of anthrax spores during stages of transformation into vegetative cells. J Bacteriol. 1966 Jul;92(1):220–228. doi: 10.1128/jb.92.1.220-228.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Rahn O. PHYSICAL METHODS OF STERILIZATION OF MICROORGANISMS. Bacteriol Rev. 1945 Mar;9(1):1–47. doi: 10.1128/br.9.1.1-47.1945_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- White T. K., Kim J. Y., Wilson J. E. Differential scanning calorimetric study of rat brain hexokinase: domain structure and stability. Arch Biochem Biophys. 1990 Feb 1;276(2):510–517. doi: 10.1016/0003-9861(90)90752-k. [DOI] [PubMed] [Google Scholar]