Abstract
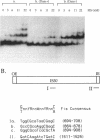
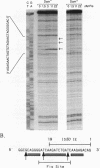
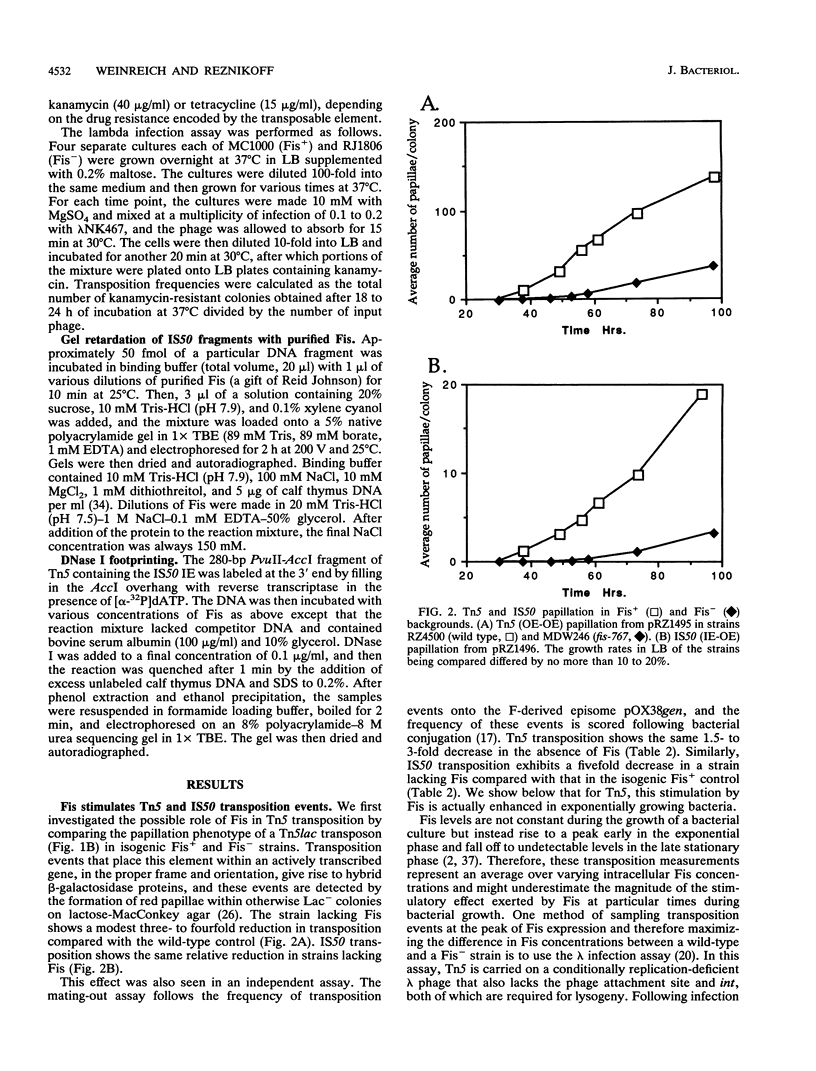
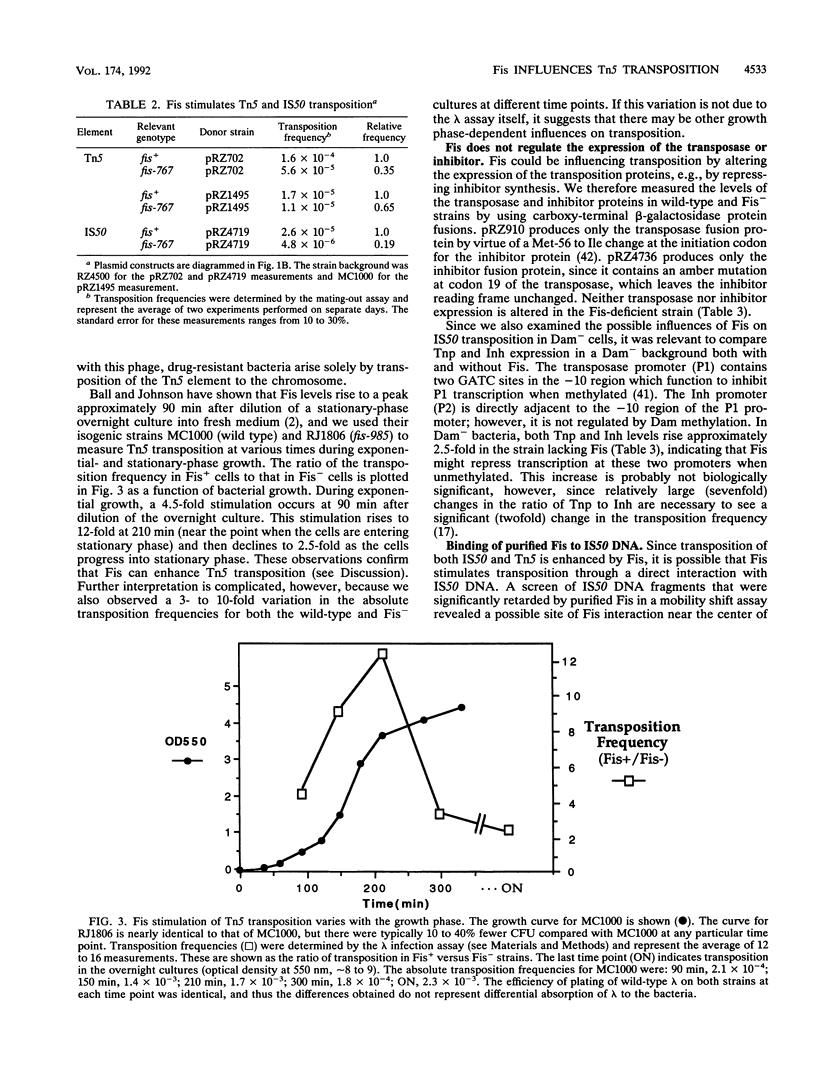
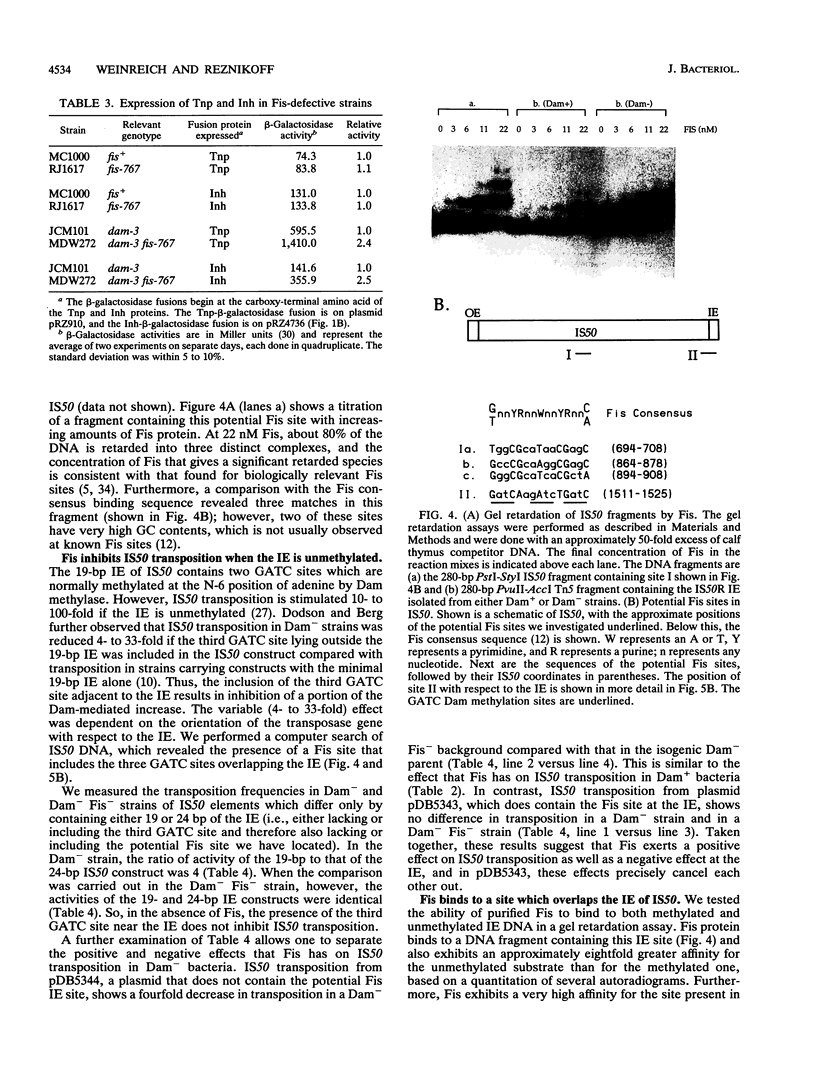
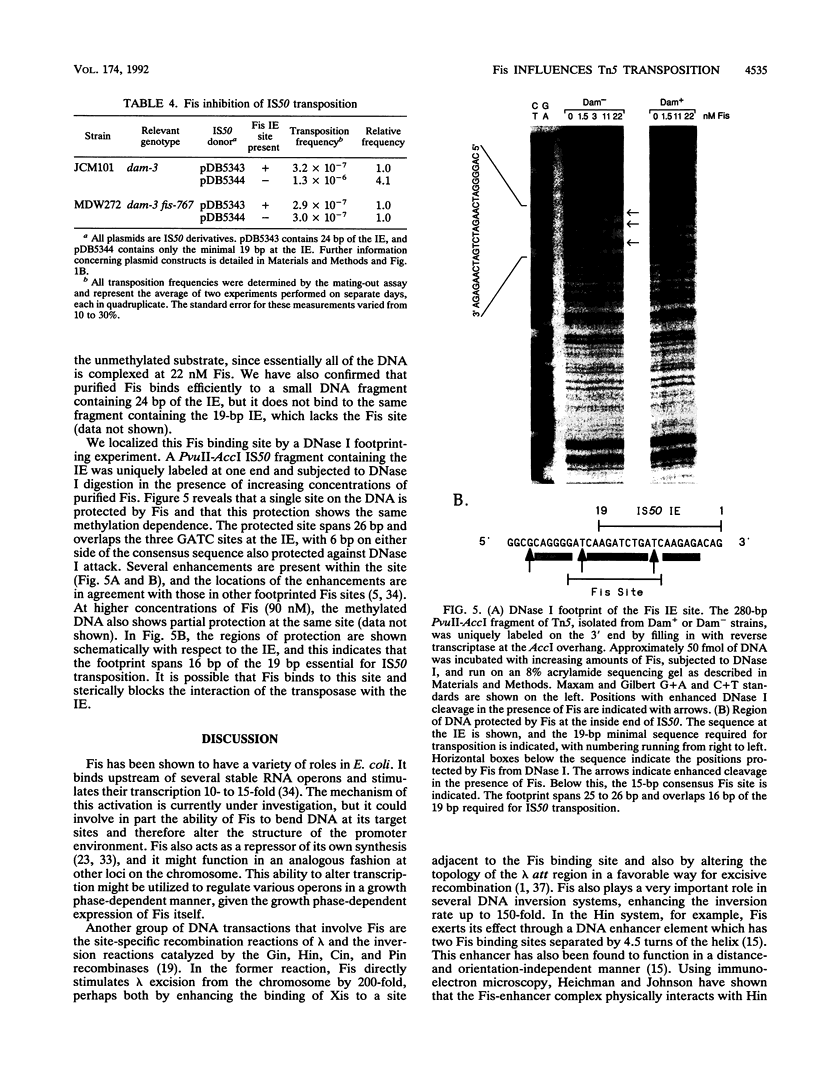
The Fis (factor for inversion stimulation) protein of Escherichia coli was found to influence the frequency of transposon Tn5 and insertion sequence IS50 transposition. Fis stimulated both Tn5 and IS50 transposition events and also inhibited IS50 transposition in Dam-bacteria. This influence was not due to regulation by Fis of the expression of the Tn5 transposition proteins. We localized, by DNase I footprinting, one Fis site overlapping the inside end of IS50 and give evidence to strongly suggest that when Fis binds to this site, IS50 transposition is inhibited. The Fis site at the inside end overlaps three Dam GATC sites, and Fis bound efficiently only to the unmethylated substrate. Using a mobility shift assay, we also identified another potential Fis site within IS50. Given the growth phase-dependent expression of Fis and its differential effect on Tn5 versus IS50 transposition in Dam-bacteria, we propose that the high levels of Fis present during exponential growth stimulate transposition events and might bias those events toward Tn5 and away from IS50 transposition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball C. A., Johnson R. C. Efficient excision of phage lambda from the Escherichia coli chromosome requires the Fis protein. J Bacteriol. 1991 Jul;173(13):4027–4031. doi: 10.1128/jb.173.13.4027-4031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball C. A., Johnson R. C. Multiple effects of Fis on integration and the control of lysogeny in phage lambda. J Bacteriol. 1991 Jul;173(13):4032–4038. doi: 10.1128/jb.173.13.4032-4038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Schmandt M. A., Lowe J. B. Specificity of transposon Tn5 insertion. Genetics. 1983 Dec;105(4):813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruist M. F., Glasgow A. C., Johnson R. C., Simon M. I. Fis binding to the recombinational enhancer of the Hin DNA inversion system. Genes Dev. 1987 Oct;1(8):762–772. doi: 10.1101/gad.1.8.762. [DOI] [PubMed] [Google Scholar]
- DeLong A., Syvanen M. Membrane association of the Tnp and Inh proteins of IS50R. J Bacteriol. 1990 Sep;172(9):5516–5519. doi: 10.1128/jb.172.9.5516-5519.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson K. W., Berg D. E. A sequence at the inside end of IS50 down regulates transposition. Plasmid. 1991 Mar;25(2):145–148. doi: 10.1016/0147-619x(91)90028-u. [DOI] [PubMed] [Google Scholar]
- Dodson K. W., Berg D. E. Factors affecting transposition activity of IS50 and Tn5 ends. Gene. 1989;76(2):207–213. doi: 10.1016/0378-1119(89)90161-3. [DOI] [PubMed] [Google Scholar]
- Heichman K. A., Johnson R. C. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science. 1990 Aug 3;249(4968):511–517. doi: 10.1126/science.2166334. [DOI] [PubMed] [Google Scholar]
- Hübner P., Arber W. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 1989 Feb;8(2):577–585. doi: 10.1002/j.1460-2075.1989.tb03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Lazaar A. L., Syvanen M. Regulation of Tn5 by the right-repeat proteins: control at the level of the transposition reaction? Cell. 1982 Oct;30(3):883–892. doi: 10.1016/0092-8674(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ball C. A., Pfeffer D., Simon M. I. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci U S A. 1988 May;85(10):3484–3488. doi: 10.1073/pnas.85.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Glasgow A. C., Simon M. I. Spatial relationship of the Fis binding sites for Hin recombinational enhancer activity. Nature. 1987 Oct 1;329(6138):462–465. doi: 10.1038/329462a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. Copy number control of Tn5 transposition. Genetics. 1984 May;107(1):9–18. doi: 10.1093/genetics/107.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. DNA sequences at the ends of transposon Tn5 required for transposition. Nature. 1983 Jul 21;304(5923):280–282. doi: 10.1038/304280a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Simon M. I. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985 Jul;41(3):781–791. doi: 10.1016/s0092-8674(85)80059-3. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Yin J. C., Reznikoff W. S. Control of Tn5 transposition in Escherichia coli is mediated by protein from the right repeat. Cell. 1982 Oct;30(3):873–882. doi: 10.1016/0092-8674(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Josaitis C. A., Gaal T., Ross W., Gourse R. L. Sequences upstream of the-35 hexamer of rrnB P1 affect promoter strength and upstream activation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):307–311. doi: 10.1016/0167-4781(90)90186-6. [DOI] [PubMed] [Google Scholar]
- Kahmann R., Rudt F., Koch C., Mertens G. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell. 1985 Jul;41(3):771–780. doi: 10.1016/s0092-8674(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Koch C., Ninnemann O., Fuss H., Kahmann R. The N-terminal part of the E.coli DNA binding protein FIS is essential for stimulating site-specific DNA inversion but is not required for specific DNA binding. Nucleic Acids Res. 1991 Nov 11;19(21):5915–5922. doi: 10.1093/nar/19.21.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrewa D., Granzin J., Koch C., Choe H. W., Raghunathan S., Wolf W., Labahn J., Kahmann R., Saenger W. Three-dimensional structure of the E. coli DNA-binding protein FIS. Nature. 1991 Jan 10;349(6305):178–180. doi: 10.1038/349178a0. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Reznikoff W. S. Use of a Tn5 derivative that creates lacZ translational fusions to obtain a transposition mutant. Gene. 1988 Mar 31;63(2):277–285. doi: 10.1016/0378-1119(88)90531-8. [DOI] [PubMed] [Google Scholar]
- Makris J. C., Nordmann P. L., Reznikoff W. S. Mutational analysis of insertion sequence 50 (IS50) and transposon 5 (Tn5) ends. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2224–2228. doi: 10.1073/pnas.85.7.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris J. C., Reznikoff W. S. Orientation of IS50 transposase gene and IS50 transposition. J Bacteriol. 1989 Sep;171(9):5212–5214. doi: 10.1128/jb.171.9.5212-5214.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommas S. A., Syvanen M. Temporal control of transposition in Tn5. J Bacteriol. 1988 Feb;170(2):889–894. doi: 10.1128/jb.170.2.889-894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Vanet A., Vijgenboom E., Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann O., Koch C., Kahmann R. The E.coli fis promoter is subject to stringent control and autoregulation. EMBO J. 1992 Mar;11(3):1075–1083. doi: 10.1002/j.1460-2075.1992.tb05146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna R., Finkel S. E., Johnson R. C. Identification of two functional regions in Fis: the N-terminus is required to promote Hin-mediated DNA inversion but not lambda excision. EMBO J. 1991 Jun;10(6):1593–1603. doi: 10.1002/j.1460-2075.1991.tb07680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Thompson J. F., Newlands J. T., Gourse R. L. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990 Nov;9(11):3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Carle G. F., Berg D. E. Sequences essential for transposition at the termini of IS50. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7293–7297. doi: 10.1073/pnas.80.23.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz V. P., Reznikoff W. S. Translation initiation of IS50R read-through transcripts. J Mol Biol. 1991 Sep 5;221(1):65–80. doi: 10.1016/0022-2836(91)80205-9. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Moitoso de Vargas L., Koch C., Kahmann R., Landy A. Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway. Cell. 1987 Sep 11;50(6):901–908. doi: 10.1016/0092-8674(87)90516-2. [DOI] [PubMed] [Google Scholar]
- Yin J. C., Krebs M. P., Reznikoff W. S. Effect of dam methylation on Tn5 transposition. J Mol Biol. 1988 Jan 5;199(1):35–45. doi: 10.1016/0022-2836(88)90377-4. [DOI] [PubMed] [Google Scholar]
- Yin J. C., Reznikoff W. S. dnaA, an essential host gene, and Tn5 transposition. J Bacteriol. 1987 Oct;169(10):4637–4645. doi: 10.1128/jb.169.10.4637-4645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. C., Reznikoff W. S. p2 and inhibition of Tn5 transposition. J Bacteriol. 1988 Jul;170(7):3008–3015. doi: 10.1128/jb.170.7.3008-3015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]