Abstract
Behavioral inhibition (BI) is a risk factor for anxiety disorders. While the two constructs bear behavioral similarities, previous work has not extended these parallels to the neural level. This study examined amygdala reactivity during a task previously used with clinically anxious adolescents. Adolescents were selected for enduring patterns of BI or non-inhibition (BN). We examined amygdala response to evocative emotion faces in BI (N=10, mean 12.8 years) and BN (N=17, mean 12.5 years) adolescents while systematically manipulating attention. Analyses focused on amygdala response during subjective ratings of internal fear (constrained attention) and passive viewing (unconstrained attention) during the presentation of emotion faces (Happy, Angry, Fearful, and Neutral). BI adolescents, relative to BN adolescents, showed exaggerated amygdala response during subjective fear ratings and deactivation during passive viewing, across all emotion faces. In addition, the BI group showed an abnormally high amygdala response to a task condition marked by novelty and uncertainty (i.e., rating fear state to a Happy face). Perturbations in amygdala function are evident in adolescents temperamentally at risk for anxiety. Attention state alters the underlying pattern of neural processing, potentially mediating the observed behavioral patterns across development. BI adolescents also show a heightened sensitivity to novelty and uncertainty, which has been linked to anxiety. These patterns of reactivity may help sustain early temperamental biases over time and contribute to the observed relation between BI and anxiety.
Keywords: Temperament, Anxiety, Attention, Face-processing, Amygdala, fMRI
Introduction
Behavioral inhibition is an early-appearing temperament marked by a tendency to withdraw or show reticence in the face of novel social situations (Kagan et al., 1988). Behaviorally inhibited children are hypervigilant during situations of uncertainty or novelty and are often labeled as “shy” by both adults and their peers (Coplan et al., 1994, Fox et al., 1995). In characterizing the construct, Kagan and colleagues (Garcia Coll et al., 1984, Kagan et al., 1984) drew extensively on work describing the structure and function of the fear circuit (Amaral, 1986, Davis et al., 1997). The constellation of behaviors comprising behavioral inhibition was hypothesized to reflect increased reactivity of the amygdala.
Research has proceeded for the last two decades based on this model using behavioral (e.g., motoric reactivity in infancy) and psychophysiological (e.g., autonomic reactivity) markers theoretically linked to amygdalar functioning (Fox et al., 2005, Pérez-Edgar and Fox, 2005). The first direct observations of amygdala activity in vivo associated with behavioral inhibition emerged in the last few years (Schwartz et al., 2003). Schwartz et al. (2003) demonstrated that young adults characterized as behaviorally inhibited in the second year of life exhibited amygdala hyper-reactivity to novel faces with neutral expressions relative to familiar faces with the same neutral expression. The authors argued that the heightened amygdala activity displayed by these adults identified as behaviorally inhibited in childhood was a result of their heightened response to novelty. These data were the first to report direct links between early behavioral inhibition and amygdala activity, laying the foundation for the current study.
Interest in behavioral inhibition has grown with the publication of reports linking early inhibition to anxiety later in life (Goldsmith and Lemery, 2000, Kagan, 2001, Kagan et al., 2001, Pérez-Edgar and Fox, 2005). For example, children of parents with panic disorder have higher levels of behavioral inhibition (Rosenbaum et al., 1988), while children high in behavioral inhibition are more likely to show anxious symptomatology (Hirshfeld et al., 1992). Behavioral inhibition and anxiety are both marked by withdrawal and avoidant behavior (Pine, 1999). Behavioral inhibition and anxiety also share core psychophysiological markers, including right frontal EEG asymmetry (Fox et al., 2001), enhanced stress response in the L-HPA axis (Schmidt et al., 1997), perturbations in heart rate and vagal tone (Marshall and Stevenson-Hinde, 1998), and enhanced startle response (Schmidt and Fox, 1999). Of note, no imaging studies to date have examined behaviorally inhibited individuals using tasks previously used to differentiate clinically anxious individuals from the general population, or vice versa. As such, we do not know if the parallels between anxiety and behavioral inhibition extend to the neural level.
McClure et al. (2007) recently demonstrated alterations in amygdala function in adolescents with anxiety disorders. As predicted, anxious adolescents showed enhanced amygdala reactivity to faces with fearful expressions during a face-processing task in the fMRI environment. This excessive amygdala response was modulated by attention: the response was evident only when attention was directed towards internal feelings of fear and could not be detected in other attention conditions. Of note, when participants were asked to simply passively view fearful faces (i.e., attention is unconstrained), the anxious adolescents exhibited significant amygdala deactivation. Furthermore, anxious adolescents showed a distinct amygdala response selectively to fearful faces (with a similar trend for angry faces) that did not extend to neutral or happy faces.
The present work sought to extend the Schwartz et al. (2003) and McClure et al. (2007) findings by examining behaviorally inhibited adolescents’ responses to evocative stimuli using the identical paradigm employed by McClure et al (2007). The cohort of behaviorally inhibited adolescents studied here had been identified in infancy and were characterized with regard to behavioral inhibition throughout preschool and childhood (Fox et al., 1995, Fox et al., 2001). This provided the opportunity to identify and examine a group of children exhibiting an enduring pattern of high levels of behavioral inhibition. This group was contrasted with a sample of non-inhibited children from the same cohort who did not display stable inhibition across development but rather typical variations in their pattern of social behavior.
The current study examined the hypothesis that adolescents characterized with behavioral inhibition would display similar neural responses to fear faces as adolescents with anxiety disorders. Based on McClure et al. (2007) we predicted (1) heightened amygdala activation in response to fearful facial expressions, when attention is focused on self-assessment of fear state, in inhibited compared to non-inhibited adolescents, and (2) amygdala deactivation in response to fearful facial expressions in passive viewing in inhibited adolescents only. In addition, based on the work of Kagan et al. (Kagan, 1997, Kagan and Snidman, 2004) and Schwartz (2003) we predict that the inhibited adolescents will show heightened amygdala activity when presented with novelty or discrepancy (e.g., Rating fear state to a Happy stimulus).
Methods
Participants
Subject Classification
Forty-four adolescents participated in the current study. The adolescents were drawn from a longitudinal study of temperament and affect regulation (Fox et al., 1995, Fox et al., 2001). This longitudinal cohort included 153 children who were selected in infancy and evaluated at ages nine months, 14 months, 24 months, four years, and seven years. At each visit, measures of temperamental and psychophysiological reactivity were collected in the laboratory (Calkins et al., 1996, Fox et al., 2001, Henderson et al., 2004).
Standardized laboratory measures of BI were collected when subjects were 14 & 24 months (Kagan et al., 1989, Fox et al., 2001), and 4 & 7 years of age (Rubin, 1989, Fox et al., 2001). These scores were standardized and then meaned to create a single, stable measure of BI over time. Higher scores reflected higher levels of BI. The adolescents in the current study were representative of the initial cohort with respect to the range of BI scores (mean BI scores: 0.05 participants vs. 0.07 cohort, t = −0.13, p = 0.90). Using K-means cluster analysis (Cairns et al., 1998), two groups were identified (Table 1). The first group (n=17) was higher on behavioral inhibition assessed in toddlerhood and social reticence assessed in early childhood than the second group (n=27). Group 1 was labeled ‘Behaviorally Inhibited’ (BI) and group 2 was labeled ‘Behaviorally Non-inhibited’ (BN).
Table 1.
Demographic and temperament measures (means and SD) for the inhibited and non-inhibited adolescents.
| All Subjects | Inhibited | Non-Inhibited | |
|---|---|---|---|
| Age | 12.63 (1.71) | 12.80 (1.69) | 12.53 (1.77) |
| Gender | 10/17 | 2/8 | 8/9 |
| IQ | 117.7 (8.78) | 119.1 (10.61) | 116.9 (7.89) |
| Inhibition at 14 months | −1.25 (2.29) | −0.04 (1.58) * | −1.98 (2.39) * |
| Inhibition at 24 months | −1.54 (4.60) | 2.84 (3.49) ** | −3.46 (3.65) ** |
| Reticence at 4 years | 0.19 (0.20) | 0.36 (0.26) ** | 0.10 (0.05) ** |
| Reticence at 7 years | 0.10 (0.07) | 0.13 (0.07) | 0.08 (0.06) |
Gender = Male/Female
p<0.05;
p<0.01
Of the 44 adolescents selected for the neuroimaging study, five declined participation and four met exclusionary criteria. Exclusionary criteria included metal in body (2 subjects), current psychoactive substance use (1 subject), and acute/unstabilized psychopathology (1 subject). Subjects with DSM-IV Axis 1 diagnoses were not excluded since increased clinical risk (particularly for anxiety) is an emerging hallmark of stable, high BI. Non-BI linked disorders that may interfere with the completion of the task (e.g., severe pervasive developmental disorder, PDD) were exclusionary. For two adolescents, technical problems prohibited data acquisition. Finally, six did not provide useable fMRI data due to either excessive movement or technical difficulties at testing. Adolescents who did and did not complete the scan were comparable in age, t(42) = 0.99, p = 0.33, gender, χ2 = 0.86, p = 0.35, and cluster assignment, χ2 = 0.83, p = 0.36, although the adolescents who were scanned had higher IQ scores (112 vs. 118), t(41) = −2.20, p = 0.03.
For the remaining 27 adolescents, 10 were in Cluster 1, the BI group (age: M = 12.6 years, SD = 1.59; IQ: M = 119.1, SD = 11.34; gender: 2 male, 8 female) and 17 were in Cluster 2, the BN group, (age: M = 12.6 years, SD = 1.75; IQ: M = 116.9, SD = 7.89; gender: 8 male, 9 female). The two temperament groups did not differ in age, t(25) = 0.08, p = 0.94, IQ, t(23) = 0.56, p = 0.58, or gender, χ2 = 3.00, p = 0.08.
Adolescents were screened for psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School Aged Children–Present and Lifetime Version (Kaufman et al., 1997), performed by an experienced clinician who exhibited satisfactory reliability on the exam with kappa > 0.75 for all diagnoses. Two BI (20%) and three BN (18%) adolescents met criteria for an Axis I diagnosis. BI adolescents were diagnosed with attention deficit hyperactivity disorder (ADHD)-inattentive type and generalized anxiety disorder (GAD; subject 1) and major depressive disorder (MDD; subject 2). BN adolescents were diagnosed with oppositional defiant disorder (ODD), conduct disorder, and specific phobia (subject 1), ADHD (subject 2), and Tourette’s syndrome (subject 3). Participants currently on psychoactive substances were excluded. To examine the impact of psychiatric diagnosis on the results, analyses were conducted with and without diagnosed adolescents. No differences emerged and thus analyses comprising the whole sample are presented here. Although not statistically significant, sex distribution differed between groups. To ensure that sex did not affect the results, it was used as a between-subjects factor in initial analyses. Since sex had no significant effects on the variables of interest (p’s > 0.31), it was removed from the analyses.
The study was approved by the institutional review boards at the National Institute of Mental Health in Bethesda, MD, and the University of Maryland in College Park, MD. All subjects and their parents provided written informed assent/consent to participate in the study.
Face Processing Task
Stimuli
Participants viewed a series of 32 adult faces (8 happy, 8 angry, 8 fearful, and 8 neutral) during the task. These 32 actors were randomly drawn from a larger pool of 56 actors for each subject. Similarly, the selection of facial expressions for each actor varied across participants so that different actors displayed different emotions to different participants. Gray-scale face stimuli were derived from Ekman and Friesen (1976), Gur (www.uphs.upenn.edu/bbl/pubs/downloads/nptasks.shtml), and Tottenham and Nelson (www.macbrain.org/faces/idex.htm). All pictures controlled for head size in frame and luminosity and were identical to those used in previous research (Monk et al., 2003, Pine et al., 2005, Rich et al., 2006, Roberson-Nay et al., 2006, McClure et al., 2007).
Procedures
Each of the 32 faces was presented four times as part of one 160-trial run using Avotec Silent Vision Glasses (Stuart, FL). Trials were divided into four 40-trial epochs (32 faces plus 8 trials presenting only a fixation point). Each epoch was further subdivided into 4 ten-trial blocks. During each block, 8 of the 32 faces and 2 fixation trials were presented in random order. Faces were presented with the constraint that two pictures of each emotion type appeared in each block and no participant viewed any actor posing more than one expression.
Participants were asked to complete one of four tasks during face presentation: (1) Participants rated the level of threat each face presented on a 5-point scale: “How hostile is this face?”. (2) Participants rated internal fear levels for each face presented on a 5-point scale: “How afraid are you of this face?”. (3) Participants rated the width of the nose on a five-point scale: “How wide is the nose?”. (4) Participants passively viewed the presented faces. These four tasks are referred to as the “hostile”, “afraid”, “nose”, and “passive” attention conditions. Ratings were recorded using a five-key button box developed by MRI Devices (Waukesha, WI). The reaction time (RT) for each rating was also noted.
At the end of the 160-trial run, each presented face had been rated on all three questions and passively viewed. Block and epoch were randomly presented across participants. For each trial, instructions were presented for 3000 ms and each face or fixation point was displayed for 4000 ms. Participants rated each face while it appeared on the screen. The interstimulus interval varied between 750 and 1250 ms. Prior to scanning, participants rehearsed the task using a practice set of faces with neutral expressions. There was no overlap between practice and experimental faces.
MRI Data Acquisition
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired on a General Electric (Waukesha, Wisconsin) Signa 3T scanner. Following sagittal localization and manual shimming, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix size of 64 X 64 mm, repetition time (TR) of 2000 milliseconds, echo time (TE) of 40 milliseconds, field of view (FOV) of 240 mm, and voxels of 2.0 X 2.0 X 2.0 mm. Images were acquired in 23 contiguous 5-mm axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC-PC) line. All functional data were gathered in a single 14-minute run for each subject. After echo-planar imaging (EPI) acquisition, a high-resolution T1-weighted anatomical image was acquired to aid with spatial normalization. A standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV = 256, number of excitations (NEX) = 1, TR = 11.4 milliseconds, TE = 4.4 milliseconds, matrix = 256 X 256, time to inversion (TI) = 300 milliseconds, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels) was used.
MRI Processing
Reconstructed fMRI images were examined for each subject for excessive motion using MedX software (Medical Numerics, Sterling, Virginia). Subjects who moved more than 1.5 mm in any plane (N=3) were discarded. All subsequent analyses were conducted with SPM software (SPM99b, Wellcome Department of Imaging Neuroscience, University College of London, London, United Kingdom) and Matlab 5.3 (Mathworks, Natick, Massachusetts) routines. Functional data were corrected for slice timing, motion corrected, coregistered to the anatomical data, spatially normalized to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99, and smoothed with an isotropic 8-mm full width at half maximum (FWHM) Gaussian kernel. After preprocessing, fMRI images were visually inspected to evaluate the quality of the normalization procedure.
We estimated event-related response amplitudes at the individual subject level for every event type (i.e., face emotion) in each attention set using the General Linear Model (GLM). We generated contrast images for each subject using pair-wise comparisons of event-related responses across event types. We then divided each contrast image by the subject-specific voxel time series means, yielding the percent fMRI signal change (Zarahn et al., 1997).
Data Analyses
Behavioral Data
Behavioral measures (ratings and RTs) were used to confirm the participants’ attention to the task and examine any group differences in perceptual responses to the stimuli. Due to an equipment malfunction, the behavioral rating and RT data for one participant was not recorded during fMRI acquisition. As such, the behavioral data analyses involved 9 BI adolescents and 17 BN adolescents.
Ratings and RTs were examined with repeated measures analyses of variance (ANOVA) using Emotion (fearful, angry, happy, neutral) and Attention (hostile, afraid, nose) as the within-subjects factor, and Temperament (BI vs. BN) as the between-subjects factor. To minimize the risk of Type 1 errors the Greenhouse-Geisser (G-G) procedure was applied to the repeated measures ANOVA when appropriate (Geisser and Greenhouse, 1958). The degrees of freedom presented in the text are not G-G corrected. However, epsilon (ε) was indicated when less than 1.0.
fMRI Data
For all group-level analyses, a random effects model was employed to permit population-level inferences (Holmes and Friston, 1998). Because the amygdala has been specifically implicated in temperamental reactivity (Kagan, 2001, Schwartz et al., 2003), and is a prime candidate for conferring risk for anxiety disorders, we restricted analyses to this structure using a region of interest (ROI) strategy. The boundaries of the amygdala were defined using standard anatomical criteria (Szeszko et al., 1999) on a single MNI template and applied to all normalized brains at the group level. We used a small volume correction (SVC) Gaussian random field threshold (α= .05) and a threshold of p corrected < 0.05 for significant findings (Friston et al., 1996, Worsley et al., 1996, Hariri et al., 2002).
The initial analysis used the contrast between subjective fear-ratings of fearful faces and passive viewing of fearful faces. This contrast allowed us to focus on the perturbations previously noted in anxious adolescents and examine the effects of temperament on the attentional modulation of processing fearful stimuli.
Post-hoc analyses of these initial findings selected the three most-active peak coordinates from the right and left amygdala identified in the contrast between fear ratings and passive viewing (see Table 2). Activation levels at these coordinates were used for all subsequent analyses. Individual peak BOLD signal changes at these coordinates were extracted from the SPM dataset for each Emotion by Attention combination versus fixation to be included in analyses using SPSS13.0. This method follows procedures established in prior studies (McClure et al., 2007) to generate a stable, distributed indicator of activation without potentially diluting the findings by averaging across the entire amygdala. The use of extracted data for all experimental conditions for analyses on the SPSS13.0 platform provides more flexibility, allowing for multifactorial analyses to be readily conducted. Thus, with this approach, we could examine the effects of temperament, attention-state, and face-emotion across the entire experimental session.
Table 2.
Amygdala activation in the face-processing task for the inhibited (BI) and non-inhibited (BN) adolescents in the contrast comparing fear ratings of fearful faces to passive viewing of fearful faces. Three coordinates were identified for both the left and right amygdala and used in the larger Emotion by Attention by Temperament ANOVA. (Significance levels reflect the corrected p-values).
| x | y | z | t | p | |
|---|---|---|---|---|---|
| Left Amygdala | −8 | −4 | −14 | 2.43 | 0.053 |
|
| |||||
| −28 | 4 | −12 | 2.38 | 0.057 | |
|
| |||||
| −28 | 4 | −18 | 2.35 | 0.061 | |
|
|
|||||
| Right Amygdala | 32 | −2 | −12 | 2.92 | 0.020 |
|
| |||||
| 32 | −8 | −10 | 2.78 | 0.026 | |
|
| |||||
| 28 | −2 | −10 | 2.66 | 0.032 | |
An initial omnibus repeated measures ANOVA examined main and interaction effects of four within-subjects factors (Attention, Emotion, Laterality, and Peak) and one between-subjects factor (Temperament). Laterality and Peak showed no significant main or interaction effects (p’s > 0.23). Therefore, the results are presented for the 3-way ANOVA using Attention, Emotion and Temperament as factors. The G-G correction was again used when appropriate. Post-hoc analyses were performed to better understand the nature of significant findings.
Finally, all analyses were also run including sex and diagnosis as between-subjects factors. No effects of sex or diagnosis emerged in these analyses. As a result, the findings are reported for the main analyses without sex or diagnosis.
Results
Behavioral Data
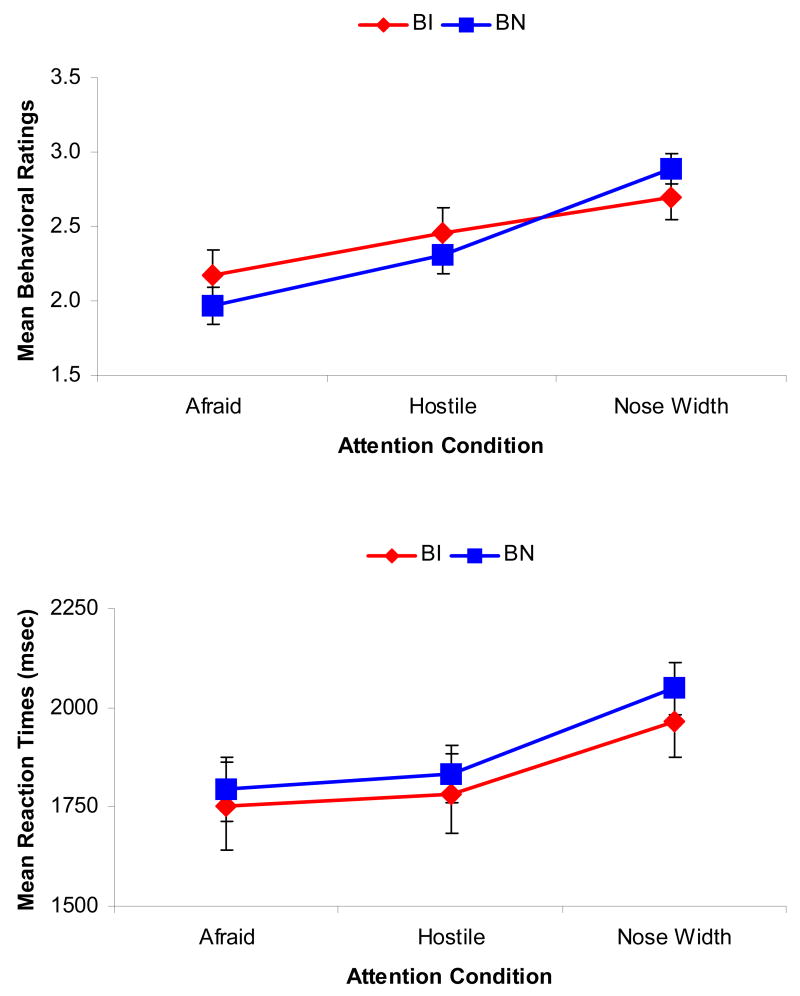
The omnibus ANOVA examining the effects of Emotion, Attention and Temperament on the 5-point subjective rating scores revealed no significant interactions involving Temperament, F’s < 2.12, p’s > 0.13 (Figure 1). This includes the omnibus three-way interaction between Emotion, Attention, and Temperament, F(6,144) = 1.32, p = 0.27, ε= 0.60, f = 0.26.
Figure 1.
Mean (a) rating scores and (b) reaction times in the face processing task: Fear ratings (“How Afraid are you”), Hostility ratings (“How Hostile is the face”), and ratings of the Nose-width (“How Wide is the Nose”) on a scale of 1 to 5.
However, there were significant main effects for Emotion and Attention, F’s > 22.89, p’s < 0.001, indicating that ratings differed significantly as a function of the depicted emotions (fearful, angry, happy, neutral) and the topic being rated (subjective fear, stimulus hostility, nose width). These main effects were subsumed by a significant Emotion by Attention interaction, F(6,144) = 40.45, p < 0.001, ε= 0.60, f = 1.30. As expected, fear and hostility ratings were highest for the Fearful and Angry faces.
A parallel analysis for reaction time found no significant effects involving Temperament, F’s < 0.90, p’s > 0.48. The ANOVA again found significant main effects for Emotion and Attention, F’s > 18.45, p’s < 0.001, which were subsumed by an Emotion by Attention interaction, F(6,144) = 10.34, p < 0.001, ε= 0.73, f = 0.66.
These results suggest that subjects properly engaged in the task, as anticipated, with no evidence of behavioral group differences in task performance. Thus, any differences in brain activation cannot be attributed to differences in behavioral performance.
Peak BOLD signal change analyses
As noted above, the initial contrast focused on amygdala activation during the fear rating (directed attention) condition versus the passive viewing (non-directed attention) condition for the fearful faces. Consistent with our hypothesis, BI adolescents showed greater amygdala activation to the directed attention condition than the non-directed attention condition relative to BN adolescents (Figure 2; Table 3).
Figure 2.
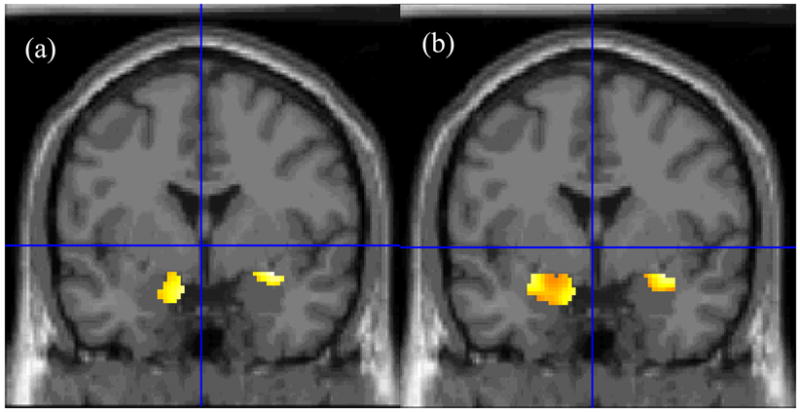
Statistical maps of voxel-wise between-group comparisons of amygdala activation during the fear rating condition relative to the passive viewing condition during the presentation of (a) fearful and (b) happy faces. BI adolescents compared to BN adolescents exhibited greater bilateral amygdala activation to both fearful and happy faces. [Coordinates for fearful faces: 32 –2 –12; happy faces: −28 –4 –12].
Subsequent analyses using SPSS 13.0 were based on the individual amygdala peak activations extracted from the SPM dataset and originating from the MNI coordinates of peak activations localized on the initial contrast.
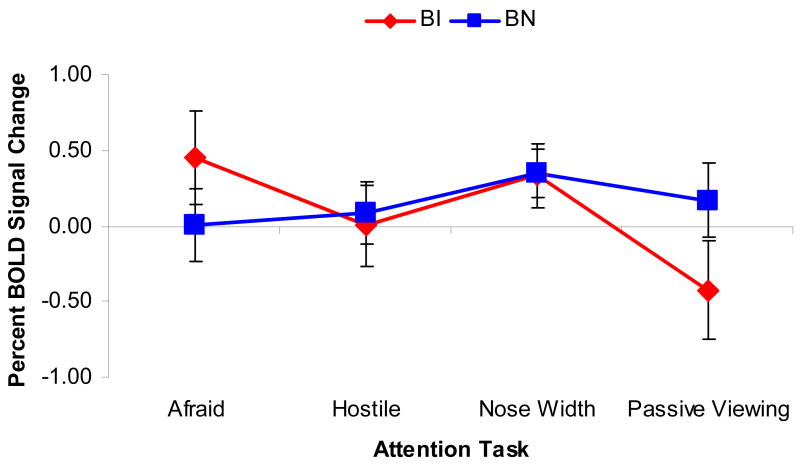
Similarly to the behavioral analysis, a full factorial ANOVA was first conducted to examine all possible main effects and interactions. The Attention by Temperament interaction was significant, F(3,75) = 2.98, p = 0.04, ε = 0.90, f = 0.35 (Figure 3).
Figure 3.
Between-group comparisons of event-elicited BOLD signal change for the amygdala during the four Attention conditions (How Afraid are you, How Hostile is the face, How Wide is the Nose, Passive Viewing). Behaviorally inhibited adolescents demonstrated deviations in amygdala activation relative to their non-inhibited peers for the fear rating and passive viewing conditions. [Analyses used the set of coordinates noted in Table 2. Results were collapsed across the Peak and Laterality within-subjects factors due to non-significance.]
This interaction reflected greater amygdala activation in the BI group, as contrasted with the BN group, during the internal fear rating, relative to other attention states, with particularly robust amygdala deactivation in the BI group during passive viewing. No group differences were found in the two other attention conditions (hostility rating or nose width ratings).
In contrast to previous findings in anxiety disorders (McClure et al., 2007), Emotion did not modulate these findings (i.e., there were no significant interactions involving Temperament and Emotion), and responses were not specific to the negative-valence faces.
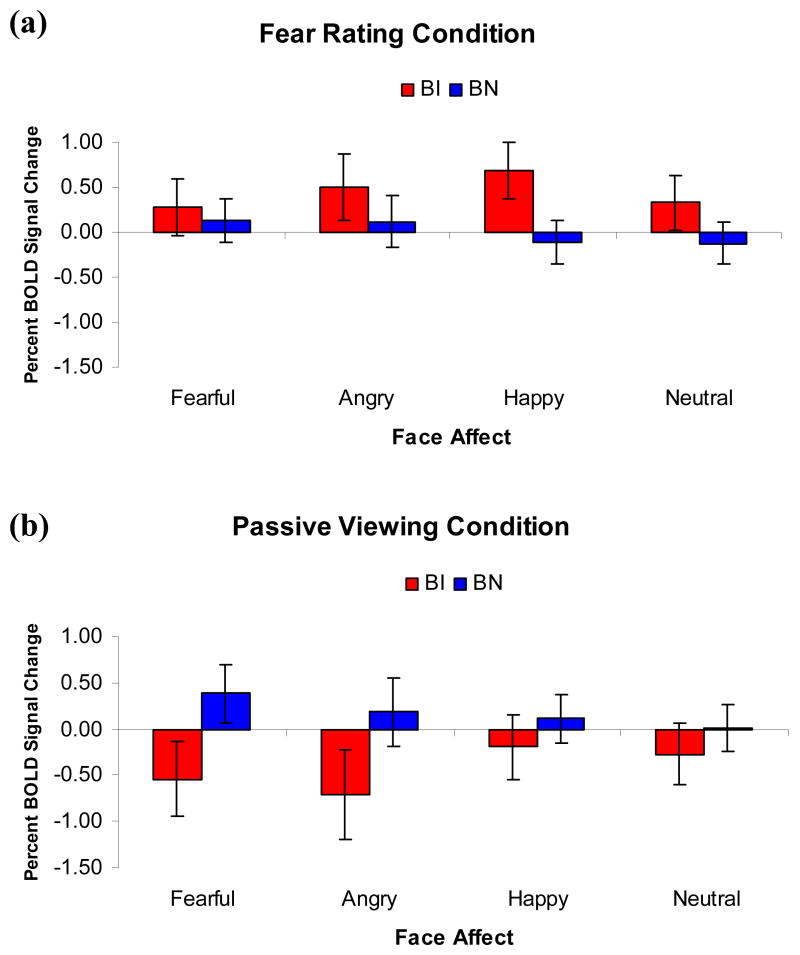
Although Emotion was not found to modulate the effects of Temperament, we conducted exploratory analyses of amygdala responses to each facial emotion, because of our strong a priori hypotheses regarding the emotion specificity of the amygdala response (Fox et al., 2005, McClure et al., 2007). Moreover, these analyses also provided insights on potential differences in activation patterns among clinically anxious and behaviorally inhibited adolescents. As expected, BI adolescents showed significant amygdala deactivation, relative to BN adolescents, when passively viewing fearful faces, t(25)=− 2.69, p=0.01, d=1.06 (Figure 4).
Figure 4.
Between group comparisons of amygdala activation for the (a) fear rating and (b) passive viewing conditions. These demonstrate a pattern of hyperactivation when rating internal fear states and deactivation when attention is left unconstrained for BI adolescents. [Analyses used the set of coordinates noted in Table 2. Results were collapsed across the Peak and Laterality within-subjects factors due to non-significance.]
The only other significant finding was that BI adolescents showed particularly high levels of amygdala activation to the happy faces when asked to report their subjective fear, t(25) = 2.61, p = 0.02, d = 1.04 (Figure 4). Potential explanations for this finding are discussed below.
Diagnostic Status
Analyses were conducted with the full sample and then repeated after excluding adolescents with any DSM-IV diagnosis. We found no changes in the results. In particular, the central Temperament by Attention interaction remained significant when observing only healthy adolescents, F(3,60) = 3.74, p = 0.02, ε = 0.88, f = 0.43.
Discussion
The current study is among the first to investigate the role temperament may play in affective processing and its modulation by attention at the neural level. Perturbations in affect and attention have been specifically implicated in the early emergence of anxiety disorders (Rauch et al., 2003), and an examination of these factors in a population at risk for anxiety may help further our understanding of the underlying etiology of this family of disorders. The data generated by the current study suggested two mechanisms underlying amygdala reactivity in behavioral inhibition: a unique attentional modulation associated with fear-related processing and a distinct response to novelty or uncertainty. We will first discuss the attention related processes evident in the findings and then turn to the role of uncertainty in the observed amygdala response.
Response to Attention State
As expected from previous work with clinically anxious adolescents (McClure et al., 2007), behaviorally inhibited adolescents, relative to non-inhibited adolescents, showed exaggerated amygdala activity when asked to rate a subjective fear state. However, counter to our hypothesis, this exaggerated response in the behaviorally inhibited group was seen across all tested emotions. In contrast, anxious adolescents showed an enhanced amygdala response selectively to fearful and angry faces, as opposed to neutral and happy faces (McClure et al., 2007). Our second hypothesis of amygdala deactivation in response to fearful faces in passive viewing was also confirmed, but, as with the first hypothesis, it was not specific to this one emotion condition.
Similar to data for adolescent anxiety disorders, the data reported here suggest that behaviorally inhibited adolescents are sensitive to the attentional constraints of a task. Although we initially assumed that this abnormal response would be specific to self-assessment of a fear state (Fox et al., 2005), this selectivity remains to be demonstrated for self-assessment of other emotional states (e.g., happy state).
The amygdala hyper-responsivity observed when attention is drawn to a fear state is consistent with the proposal of a central role for the fear circuit in behavioral inhibition (Kagan et al., 1984, Fox et al., 2005). Given that adolescents were grouped based on behaviors observed years earlier, the findings also support the proposed contribution of the fear circuit to the long-term maintenance of the behavioral and affective patterns typical of behavioral inhibition (Fox et al., 2005, Pérez-Edgar and Fox, 2005).
The unique finding in this study, relative to prior studies both in anxious and healthy adolescents, lies in the generalized hyper-responsivity of the amygdala, impervious to stimulus valence. Longitudinal studies of behavioral inhibition are critical to test whether this generalized response becomes selective to fearful stimuli when an anxiety disorder emerges. This switch could help delineate the neural mechanisms that differentiate markers of risk from those of overt disorders. Alternatively, the expression of anxiety disorders in adolescents previously identified as behaviorally inhibited may be distinct from anxiety that emerges through other routes.
Our second attention-related finding, amygdala deactivation during passive viewing, was also relatively insensitive to facial expression, again in contrast to the fear selectivity seen in anxious children (McClure et al., 2007). Amygdala deactivation has been reported previously in both adults (Davis and Whalen, 2001, Britton et al., 2005, Phan et al., 2006) and children (Thomas et al., 2001) during the presentation of evocative stimuli. Amygdala deactivation may indicate a covert and rapid decrease in neuronal activity (Petrovic et al., 1999, Petrovic et al., 2004, Shmuel et al., 2006), which could help to prevent evocative stimuli from interfering with functioning. Inhibited adolescents may be particularly efficient at diverting processing resources away from evocative stimuli when not explicitly asked to attend to these stimuli (e.g., passive viewing). This may be in line with recent work suggesting little to no amygdalar response, or even a reduction in amygdala response, when attention is explicitly diverted from evocative stimuli (Pessoa et al., 2002, Pessoa et al., 2005). Whether this avoidant response is automatic and a biological component of inhibited temperament or a learned and adaptive mechanism will need to be further examined.
Response to Uncertainty and Ambiguity
As hypothesized, behaviorally inhibited adolescents showed abnormally high levels of amygdala reactivity during an ambiguous condition (i.e., rating subjective fear to a happy face). Kagan (Kagan et al., 1984, Kagan, 1997, Kagan and Snidman, 2004) has proposed that a sensitivity to novelty, uncertainty, or ambiguity is a central component of behavioral inhibition temperament profile. This sensitivity may underlie the unique pattern of reactivity seen in behaviorally inhibited adolescents. Since happy faces are by definition chosen as non-threatening stimuli, the instruction to rate one’s fear state may produce a novel cognitive state marked by uncertainty or ambiguity.
The amygdala has been shown to respond not only to negative stimuli, but also to arousing stimuli, whose salience is linked to conflicting, novel or rewarding features (Baxter et al., 2000, Baxter and Murray, 2002). This is in line with the contention that amygdala reactivity to fearful faces is linked to the ambiguous or uncertain information provided by the stimulus (Whalen, 1998). The inability to tolerate uncertainty has been implicated as a neurocognitive correlate of anxiety (Dugas et al., 2004) and recent work suggests a developmental link to the emergence of the disorder (Krain et al., 2006). Behavioral inhibition has also been linked to a distinct pattern of response to novelty or uncertainty.
Adolescents in the current study were selected in infancy for varying patterns of reactivity to novel auditory and visual stimuli (Fox et al., 2001). The present finding suggests that this characteristic present in infancy may be enduring and manifest in the form of heightened amygdala responsivity to unusual events.
The unique neural sensitivity of behaviorally inhibited children to conflicting situations is also supported by an earlier report in an independent sample of 7- to 12-year-olds from the same longitudinal cohort used here (Bar-Haim et al., 2003). Bar-Haim and colleagues found unique patterns of early neurophysiological responses to auditory discrepancies (mismatch negativity, MMN) in the form of reduced MMN amplitudes and longer MMN latencies in the socially withdrawn children compared to their more sociable peers. Similarly, Woodward (2002) found that young children with higher levels of negative reactivity in infancy (much like the behaviorally inhibited adolescents in the current study) demonstrated larger ERP responses in the Nc component to oddball and invalid novel stimuli in a visual paradigm. Nc responses are thought to reflect the process of novelty detection (Reynolds and Richards, 2005). In line with Kagan, Woodward suggested that highly reactive children have a lower cortical threshold for detecting and responding to novelty, leading to increased ERP amplitudes to unfamiliar events.
Also consistent with distinct brain sensitivity to novelty in this population, the fMRI study by Schwartz and colleagues (Schwartz et al., 2003) reported greater amygdala activation to novel, but emotionally neutral, faces in young adults characterized by high levels of behavioral inhibition in their second year of life compared to non-inhibited young adults.
Future Directions
A number of suggestions for future work arose from this study. First, a larger sample size would be helpful to allow for fine-grain analysis of potential moderating factors. For example, five of the adolescents (2 BI, 3 BN; all unmedicated at time of testing) were diagnosed with DSM-IV disorders. The heterogeneity of the diagnoses, along with the relatively small n, precluded any additional examination of the link between temperament, affective processing, and psychopathology. However, findings were unchanged when the adolescents with clinical diagnoses were removed from the analysis, suggesting that the findings were not driven by the presence of psychopathology.
Second, additional attention and contextual conditions need to be included to fully test the role of attention in behavioral inhibition: Self-assessment of an affective state different from fear (e.g., calm, happy, angry) would help us to clarify how task parameters affect amygdala activity. Indeed, recent work in shy college students (Hardin et al., 2006) and behaviorally inhibited adolescents (Guyer et al., 2006), suggests that inhibition is also marked by perturbations in the processing of positively valenced stimuli. These previous findings occurred in the context of reward-related paradigms, where enhanced behavioral responses occurred to rewarding stimuli (Hardin et al., 2006) and exaggerated striatal activation during monetary reward anticipation in an incentive delay task (Guyer et al., 2006). These studies reflect enhanced responsivity to rewarding stimuli in shyness or behavioral inhibition.
Third, a direct comparison and manipulation of fear state and ambiguity would allow us to assess the relative strength and interaction between the proposed mechanisms. Systematic observations would also allow us to document the link between these mechanisms and developmental outcomes linked to behavioral inhibition.
Fourth, the direct comparison of behaviorally inhibited adolescents with anxious adolescents is critical to validate the interpretation of our findings. Even more informative, would be to study inhibited children before and after the onset of an anxiety disorder in longitudinal studies.
Conclusion
Taken together, the present findings revealed a distinct pattern of amygdala responses in behaviorally inhibited adolescents to evocative stimuli as a function of attention state and task uncertainty. This pattern bears common and distinct characteristics compared to findings in anxious patients.
First, attention manipulations produce broad shifts in reactivity that do not appear to be influenced by the nature of stimulus emotion. This is in contrast to the specificity to fear evident in anxious adolescents (McClure et al., 2007). Second, behavioral inhibition may be marked by a particularly acute sensitivity to uncertainty or novelty. Intolerance to uncertainty is also evident in anxious adolescents (Krain et al., 2006). As such, profiles of amygdala activation may serve to distinguish temperamentally at risk children from psychiatrically affected peers, impacting both our current conceptualization of temperament itself and its relevance to the study and treatment of early anxiety. The current data indicate that distinct patterns of limbic response to evocative faces may not be limited to individuals with an acute mood or anxiety disorder and may instead characterize an underlying vulnerability to anxiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG. Amygdalohippocampal and amygdalocortical projections in the primate brain. Advances In Experimental Medicine And Biology. 1986;203:3–17. doi: 10.1007/978-1-4684-7971-3_1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA, Schorr EA, Gordon-Salant S. Mismatch negativity in socially withdrawn children. Biological Psychiatry. 2003;54:17–24. doi: 10.1016/s0006-3223(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Bergman LR, Kagan J, editors. Methods and models for studying the individual. Sage Publications, Inc; Thousand Oaks, CA, US: 1998. [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions Of The Royal Society Of London Series B, Biological Sciences. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Buhr K, Ladouceur R.Heimberg RG, et al.2004The role of intolerance of uncertainty in etiology and maintenance Generalized anxiety disorder: Advances in research and practice Guilford; New York: 143–163. [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting psychology press; Palo Alto CA: 1976. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review Of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1771–1784. [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Garcia Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Development. 1984;55:1005–1019. [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box’s results on the use of the F distribution in multivariate analysis. Annals of Mathematical Statistics. 1958;29:885–891. [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biological Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal function alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, Ernst M. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences. 2006;40:699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75:236–250. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:S754. [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Development. 1997;68:139–143. [PubMed] [Google Scholar]
- Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Holman SG, DiBartolo PM, editors. From Social Anxiety to Social Phobia: Multiple Perspectives. Allyn & Bacon; Needham Heights, MA, US: 2001. pp. 216–234. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kagan J, Reznick JS, Gibbons J. Inhibited and uninhibited types of children. Child Development. 1989;60:838–845. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. The long shadow of temperament. Belknap Press of Harvard University Press; Cambridge, Massachusetts: 2004. [Google Scholar]
- Kagan J, Snidman N, McManis M, Woodward S. Temperamental contributions to the affect family of anxiety. The Psychiatric Clinics of North America. 2001;24:677–688. doi: 10.1016/s0193-953x(05)70257-4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Krain AL, Hefton S, Pine DS, Ernst M, Castellanos FX, Klein RG, Milham MP. An fMRI examination of developmental differences in the neural correlates of uncertainty and decision-making. Journal of Child Psychology and Psychiatry. 2006;47:1023–1030. doi: 10.1111/j.1469-7610.2006.01677.x. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Developmental Psychobiology. 1998;33:283–292. [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm SJ, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. Journal of Cognitive Neuroscience. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Phan K, Britton J, Taylor S. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006:63. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Pine DS. Pathophysiology of childhood anxiety disorders. Biological Psychiatry. 1999;46:1556–1566. doi: 10.1016/s0006-3223(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, Mannuzza S, Moulton JL, Lissek S, Guarding M, Woldehawariat G. Face-emotion processing in offspring at risk for panic disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals Of The New York Academy Of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, Attention, and Recognition Memory in Infancy : An Event-Related Potential and Cortical Source Localization Study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent Major Depressive Disorder: An fMRI study. Biological Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Gersten M, Hirshfeld DR, Meminger SR, Herman JB, Kagan J, Reznick JS, Snidman N. Behavioral inhibition in children of parents with panic disorder and agoraphobia: A controlled study. Archives of General Psychiatry. 1988;45:463–470. doi: 10.1001/archpsyc.1988.01800290083010. [DOI] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) University of Waterloo; 1989. [Google Scholar]
- Schmidt LA, Fox NA. Conceptual, biological, and behavioral distinctions among different categories of shy children. In: Schmidt LA, Schulkin J, editors. Extreme fear, shyness, and social phobia: Origins, biological mechanisms, and clinical outcomes. Oxford University Press; New York: 1999. [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area VI. Nature Neuroscience. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: The initial neuroimaging studies of the human amygdala. Current directions in psychological science. 1998;7:177–188. [Google Scholar]
- Woodward SA. A longitudinal study of infant temperament and brain processing in childhood. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2002;63:2093. [Google Scholar]
- Worsley KM, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. NeuroImage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]