Abstract
We generated a mouse model (cKO) with a conditional deletion of TGF-β signaling in the retinal neurons by crossing TGF-β receptor I (TGF-β RI) floxed mice with nestin-Cre mice. Almost all of the newborn cKO mice had retinal detachment at the retinal pigment epithelium (RPE)/photoreceptor layer junction of the neurosensory retina (NSR). The immunostaining for chondroitin-6-sulfate showed a very weak reaction in cKO mice in contrast to intense staining in the photoreceptor layer in wild-type mice. Macroscopic cataracts, in one or both eyes, were observed in 50% of the mice by six months of age, starting as early as the first month after birth. The cKO mouse model demonstrates that the TGF-β signaling deficiency in retinal cells leads to decreased levels of chondroitin sulfate proteoglycan in the retinal interphotoreceptor matrix. This in turn causes retinal detachment due to the loss of adhesion of the NSR to RPE.
Keywords: TGF-β receptor I, retinal detachment, extracellular matrix, chondroitin-6-sulfate, Cre-lox P system, cataracts, TGF-β, retina, retinal pigment epithelium
Introduction
Retinal detachment, separation of the neurosensory retina from the underlying retinal pigment epithelia (RPE), may cause serious complications resulting in blinding diseases [1,2]. Adhesion of the neurosensory retina to the RPE is achieved by the intimate interaction of the microvilli on the apical surface of the RPE with the outer segments of the photoreceptor cells [3,4]. The virtual space between RPE and photoreceptor cells called subretinal space remains closely tight in normal subjects [1,3]. Adhesion of the neurosensory retina to RPE is regulated by active transport of fluids from the retina to the choroid through RPE and by the presence of mucopolysaccharides and extracellular matrix components that act as a glue [3,4,5]. This retinal adhesion is essential for normal retinal functions and visual processing. A variety of pathological conditions may result from the expansion of subretinal space and detachment of the neurosensory retina from RPE [1,2,3]. Retinal detachment may lead to permanent loss of vision if not reattached quickly by surgical repair [1].
Transforming growth factor-β (TGF-β), a multifunctional cytokine, regulates cell proliferation, differentiation, and extracellular matrix synthesis [6-8]. The key roles of TGF-β and its receptors in retinal fibrosis in proliferative retinal disorders and in macular degeneration are well documented (8). In mammals there are three isoforms of TGF-β designated as TGF-β 1, 2, and 3, with many overlapping physiological functions and some distinct developmental roles [7,9,10]. TGF-β is generally secreted by the cells in an inactive (latent) form, that has to be activated by proteolytic cleavage. The binding of active form of TGF-β to TGF-β receptor II initiates phosphorylation of TGF-β receptor I. Subsequent phosphorylation of Smad proteins and translocation to the nucleus results in the transcriptional activation of specific target genes [11]. One of the major roles of TGF-β is induction of synthesis of extracellular matrix components such as collagens, fibronectin, and chondroitin sulfate proteoglycans [6,8,12].
In this study, conditional gene targeting using the Cre-lox P system was implemented for disruption of the TGF-β RI gene in neuronal cells to elucidate the role of TGF-β in retinal adhesion to RPE. We made TGF-β RI conditional knockout (cKO) mice by crossing nestin-Cre mice with TGF-β RI floxed mice. Nestin is an intermediate filament gene expressed in neuronal cells. Since nestin is expressed in retinal neuronal cells from embryonic day (E) 12.5 [13], the Cre enzyme should be active from that time in nestin-Cre mice. Expression of TGF-β RI in the retina starts at E14 [9, 14]. Thus, our strategy should render TGF-β RI nonfunctional in retinal cells just before its expression starts in the cKO mice. Our findings indicate that functional loss of TGF-β RI in retinal cells results in retinal detachment.
MATERIALS AND METHODS
Transgenic mice
Nestin-Cre mice (MF1 strain) [15] were kindly provided by Dr. Ryoichiro Kagayama (Institute of Virus Research, Kyoto University, Kyoto, Japan). Generation and characterization of TGF-β RI floxed (TGF-β RIf/f) mice (C57Bl/6 X 129SvJ strain) were previously reported [9]. Nestin-Cre mice were crossed with the TGF-β RIf/f mice to generate Nestin-Cre;TGF-β RIf/+. Nestin-Cre;TGF-β RIf/+ mice were crossed with TGF-β RIf/f to generate Nestin-Cre;TGF-β RIf/f (cKO). Nestin-Cre mice were also crossed with LacZ reporter transgenic mice (pcAct-XSTOPX-lacZ) for functional analysis of Cre expression.
PCR analyses
DNA was isolated from tail biopsies using standard protocols. PCR analysis for the Cre gene and TGF-β RI floxed alleles were carried out as described [9,16]. Cre expression was analyzed by determining LacZ activity using a standard staining protocol as described [16].
Preparation of eyes for histology
Mice were anesthetized with an intraperitoneal injection of avertin (150 mg/kg). The mouse eyes were fixed with 4% paraformaldehyde for 24 hours at 4°C and equilibrated in 20% sucrose for at least two days for frozen sections. Some of the eyes were embedded in paraffin for H&E and Masson trichrome staining, before equilibrating in 20% sucrose. Those fixed for frozen tissues were embedded in OCT compound and frozen in 2-methyl butane on dry ice.
Immunostaining
The sections (20 μm thick) were cut in a cryostat and incubated overnight at 4°C with primary antibody and 3% bovine albumin, followed by FITC and TRTIC secondary antibodies. Antibodies and dilutions employed for immunostaining were the following: polyclonal anti-TGF-β RI antibody V-22 (1:100) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), monoclonal anti-chondroitin-6-sulfate antibody (1:50) (Seikagaku Co., Tokyo, Japan), and neuronal-specific enolase (1:100) (Chemicon International Inc., Temecula, CA). Double staining was performed by FITC and TRTIC secondary antibodies to TGF-β RI and neuronal-specific enolase, respectively.
RESULTS AND DISCUSSION
We made a transgenic mouse model for the conditional knockout (cKO) of TGF-β RI in retinal neuronal cells by crossing nestin-Cre and TGF-β RI floxed mice. Postnatal lethality was not observed in this mouse, as expected, suggesting that loss of TGF-β activity due to nonfunctional TGF-β RI is restricted to the retina in the eye. Retinal detachment was observed in these mice by the age of postnatal day 2. Since TGF-β RI is essential for the action of all three isoforms of TGF-β, this model gives conclusive evidence for the role of TGF-β in retinal adhesion in the eye.
PCR analyses were performed to confirm the expression of TGF-β RI flox and Cre transgenes in cKO mice (see Supplemental Fig. 1A, B). Staining for LacZ expression in the nestin-Cre mice crossed with LacZ reporter mice demonstrated a positive reaction in the retina (see Supplemental Fig. 1C). Intense staining was noted in the neuronal layers within the retina, confirming the presence of nestin (or Cre) in the retina. By using double staining for neuronal-specific enolase and TGF-β RI, we confirmed the absence of TGF-β RI protein in the retinal layers of cKO but not in wild-type (WT) mice (see Supplemental Fig.1D, E, F, G). Positive reaction for neuronal-specific enolase was observed in both WT and cKO mice (see Supplemental Fig. 1D, F). Retinal layers were disorganized in cKO mice because of retinal detachment and/or degeneration, as seen in these panels. Antibodies to TGF-β RI exhibited positive reactivity in WT mice, while in cKO mice, no positive staining was observed (see Supplemental Fig. 1E, G). The absence of TGF-β. RI in the neuronal-specific, enolase-positive retinal cells in TGF-β RI f/f; nestin-Cre cKO mice indicates a loss of TGF-β signaling activity. Immunostaining with antibodies to phosphorylated Smad 2/3, members of the TGF-β signaling pathway, also showed a negative reaction, confirming the presence of nonfunctional TGF-β RI in the cKO mouse retina (data not shown).
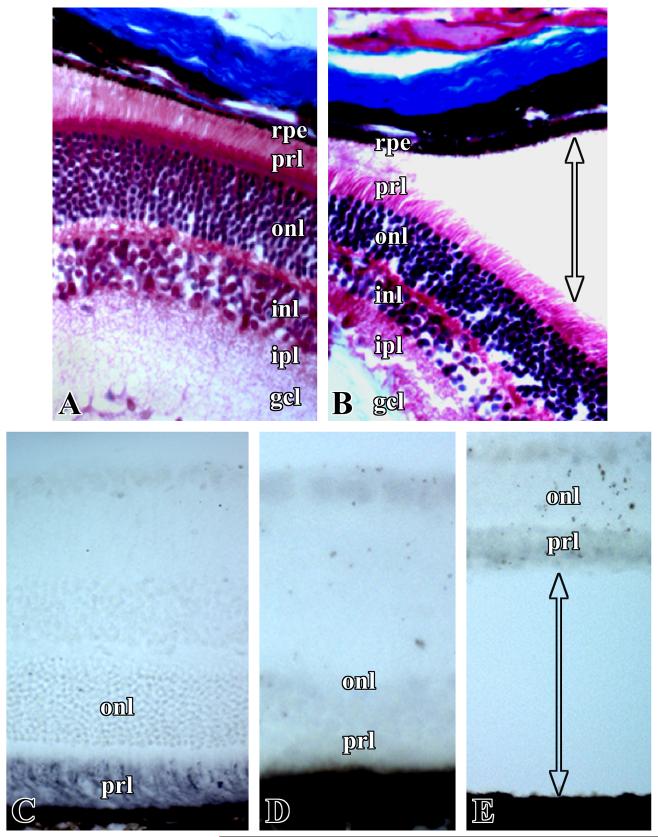
Histopathology of the eye sections showed retinal detachment in almost all of the cKO mice at birth. Neither the control mice (TGF-β RIf/f) nor TGF-β RIf/+;Nestin-Cre mice displayed retinal detachment or any other phenotype except cataracts, which developed in 50% of TGF-β RI cKO mice by the age of 6 months. At E14.5, WT and cKO mouse eye sections stained with H &E look almost identical (Fig. 1A, D). Retinal detachment was observed starting from E18.5, and the separation was clearly evident at the RPE and photoreceptor layer junction of the neurosensory retina at postnatal day 2 (Fig. 1E, F). There was also a loss of photoreceptor outer segments. In contrast, retinal layers in WT mice were intact and were attached to the RPE (Fig. 1B, C). We performed a TUNEL assay to detect apoptotic cells in the retinal sections of cKO mice, but could not detect any differences between WT and cKO mice (data not shown). These results suggest that apoptotic processes are not involved in retinal detachment in the TGF-β RI cKO mice.
Figure 1.
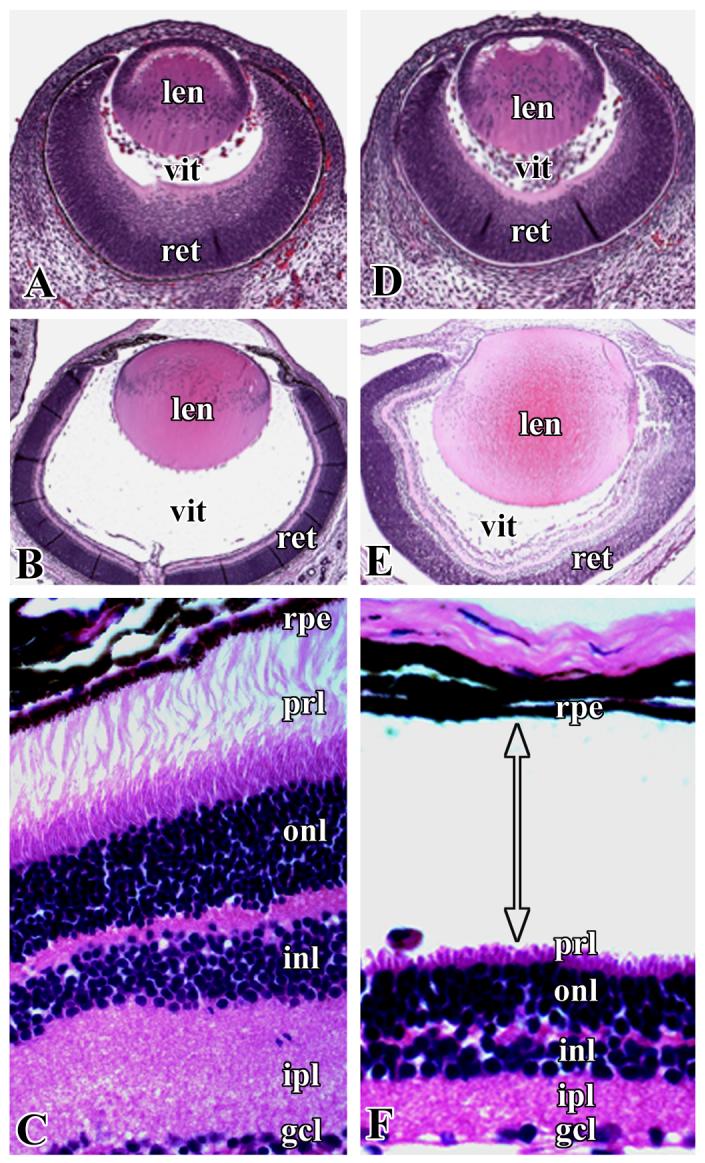
Representative pictures of H&E stained sections of the eyes of wild-type (A, B, C) and cKO (D, E, F) mice. A and D, embryonic age 14.5 days; B and E, postnatal day 2; C and F, retinal sections of wild-type and cKO mice, respectively, shown at higher magnification to illustrate retinal detachment at the pigment epithelia and photoreceptor layer. len- lens, vit- vitreous, ret- retina, rpe- retinal pigment epithelium, prl- photoreceptor layer, onl- outer nuclear layer, inl- inner nuclear layer, ipl- inner plexiform layer, gcl- ganglion cell layer. Arrow with double heads indicates the position of retinal detachment.
Previous studies with TGF-β1 and -β3 knockout mice did not show any retinal abnormalities [7]. Retinal hypercellularity was reported in TGF-β2 knockout mice (E18.5), suggesting the critical role of TGF-β2 in programmed cell death during retinal development (10). The lack of significant effects on the retina in TGF-β1 and-β3 knockout mice is likely due to the compensatory effects of the other TGF-β isoforms. No conclusive results were obtained with TGF-β RI and RII knockout mice because of embryonic lethality at E10.5 [7, 9]. Consistent retinal detachment, at the RPE/photoreceptor layer junction has been reported in TGF-β2 and-β3 double-deficient mice, which exhibit embryonic lethality at E15.5 [17]. Since these mice showed multiple abnormalities in the eyes, including a thick retina and thinner cornea and lens epithelial layer, retinal detachment could be due to complex pathological mechanisms.
Masson trichrome staining of WT and cKO mouse eye retinal sections showed no clear differences in the presence of collagen fibrils and other connective tissue components (Fig. 2A, B). However, immunostaining for chondroitin sulfate showed an intense positive reaction in the photoreceptor layer of the WT mice, but no staining was observed in cKO mice (Fig. 2C, D, E). An absence of chondroitin sulfate was observed in the eye sections of all cKO mice that had retina already detached as well as in retina still in contact with the RPE. The eye sections of the cKO mice that did not yet display retinal detachment had significantly reduced staining of chondroitin sulfate.
Figure 2.
Representative photographs of retinal sections from wild-type (A, C) and cKO (B, D, E) mice at postnatal day 2. Masson trichrome-staining (A, B) and immunostaining for chondroitin-6-sulfate with polyclonal antibody (C, D, E). Labels in the figures are similar to those shown in Figure 2.
The absence or highly reduced amount of chondroitin sulfate in the photoreceptor layer of TGF-β RI cKO mice indicates the critical role of TGF-β in the synthesis of chondroitin sulfate proteoglycans as well as the involvement of proteoglycans in retinal attachment to RPE. Chondroitin sulfate proteoglycans are the major components of the interphotoreceptor matrix (IPM) present in the subretinal space located between the retinal photoreceptor layer and RPE [4,5,17]. This matrix acts as a cementing substance to keep the neurosensory retina attached to RPE [4,5,18]. Retinal detachment was observed after intravitreal injections of xyloside, a sugar that inhibits chondroitin sulfate proteoglycan synthesis, in Yucatan micropigs [19]. A number of other studies have also provided evidence for the role of IPM chondroitin sulfate proteoglycans in retinal adhesion [3, 5,18]. The reported synthesis of proteoglycans by embryonic neural retinal cells and photoreceptors [20,21] provides support for the synthesis of chondroitin sulfate by retinal cells in our transgenic mouse model. TGF-β is well known to enhance the synthesis of extracellular matrix components like proteoglycans, collagen, and fibronectin [6,8,12]. Moreover, chondroitin sulfate has been shown to regulate neural patterning in the retina [22], suggesting its role both in retinal adhesion and retinal development.
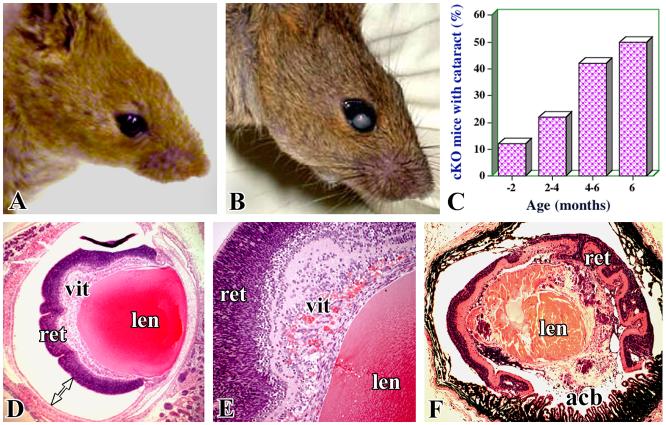
Macroscopic cataracts were observed in cKO mice beginning at an early postnatal age and cataracts development grew progressively with age. About 50% of the cKO mice exhibited cataracts in one or both eyes by the age of six months (Fig. 3A, B, C). We believe that if these mice are maintained for longer periods of time, most of the mice may develop cataracts. Macroscopic cataracts were not observed in the control mice (TGF-β RIf/f) or TGF-β RIf/+;Nestin-Cre mice (Fig. 3A). Retinal detachment appears to result in abnormal retinal architecture due to disorganization and/or degenerations of retinal layers (Fig. 3D, E). As a consequence, serious inflammatory reactions appear to be initiated with accumulation of infiltrating immune cells in the vitreous cavity (Fig. 3D, E). Also, in some cKO eye sections, red blood cells were seen, probably due to bleeding from vascular damage. As a result of the progression of these severe pathological conditions, the eyeball looks smaller in size and has a highly disorganized retina, lens, and anterior segment (Fig. 3F). Chronic retinal detachment and consequent immunopathological phenomena due to infilterating inflammatory cells may injure the lens, leading to cataractogenesis. The various chronological events and mechanisms associated with cataract development in cKO mice are not very clear. In humans, long-standing detachment of the retina is shown to lead to cataract formation due to inflammation [1,23,24]. The development of cataracts following chronic retinal detachment observed in cKO mice compliments the observations made in patients with retinal detachment [1,24].
Figure 3.
Cataract development in cKO mice. Representative pictures of wild-type (A) and cKO (B) mice show the macroscopic cataracts. (C) The incidence of macroscopic development of cataracts in one or both eyes in cKO mice in relation to age. Representative photographs of eye sections of cKO mice illustrate retinal detachment (D) presence of inflammatory and blood cells in the vitreous (E), and the disorganized appearance of a cataractous lens (F). acb- anterior chamber, other labels are similar to those indicated in Figure 2.
In summary, the results from our TGF-β RI cKO mice suggest that adhesion between the neurosensory retina and RPE is dependent on the presence of chondroitin-6-sulfate proteoglycans in the IPM in subretinal space. Since TGF-β is one of the primary regulators of proteoglycan synthesis, functional TGF-β and its receptors are critical for retinal adhesion.
Supplementary Material
Supplemental Figure 1. Characterization of TGF-β RI f/f: Nestin-Cre conditional knockout (cKO) mice. PCR analyses of mouse tail DNA for the expression of TGF-β RI floxed (A) and Cre (B) transgenes. LacZ staining of the retinal section from transgenic mice generated by crossing nestin-Cre mice and LacZ reporter mice (C). Immunostaining of retinal sections from wild-type (D, E) and cKO (F, G) mice with anti-neuronal-specific enolase antibody in red (D, F) and anti-TGF-β RI antibody in green (E, G). (Bar=10 μm)
Acknowledgements
We thank Dr. Ryoichiro Kagayama, Institute of Virus Research, Kyoto University, Japan, for providing nestin-Cre mice. This research was supported [in part] by the Intramural Research Program of the National Institutes of Dental and Craniofacial Research and National Eye Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Regillo CD, Benson WF. Retinal detachment: Diagnosis and management. Lippencott-Raven; Philadelphia: 1998. [Google Scholar]
- [2].Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16:411–421. doi: 10.1038/sj.eye.6700197. [DOI] [PubMed] [Google Scholar]
- [3].Marmor MF. Mechanisms of retinal adhesion. Prog. Ret. Res. 1993;12:179–204. [Google Scholar]
- [4].Inatani M, Tanihara H. Proteoglycans in retina. Prog. Ret. Res. 2002;21:429–427. doi: 10.1016/s1350-9462(02)00009-5. [DOI] [PubMed] [Google Scholar]
- [5].Hageman GS, Marmor MF, Yao XY, Johnson LV. The interphotoreceptor matrix mediates primate retinal adhesion. Arch. Ophthalmol. 1995;113:655–660. doi: 10.1001/archopht.1995.01100050123041. [DOI] [PubMed] [Google Scholar]
- [6].Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular marix chondriotin/dermatan sulfate proteoglycans. J. Biol. Chem. 1988;263:3039–3045. [PubMed] [Google Scholar]
- [7].Kulkarni AB, Thyagarajan T, Letterio JJ. Function of cytokines within the TGF-β superfamily as determined from transgenic and gene knockout studies in mice. Curr. Mol. Med. 2002;2:303–327. doi: 10.2174/1566524024605699. [DOI] [PubMed] [Google Scholar]
- [8].Weidmann P. Growth factors in retinal diseases: Proliferative Vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv. Ophthalmol. 1992;36:373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- [9].Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGF beta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng X-H, Derynck R. Specificity and versality in TGF-β signaling through smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- [12].Tiedmann K, Olander B, Eklund E, Todorova L, Bengtsson M, Maccarana M, Westergren-Thorsson G, Malmstrom A. Regulation of the chondoitin/dermatan fine structure by transforming growth factor-beta1 through effects on polymer-modifying enzymes. Glycobiology. 2005;15:1277–1285. doi: 10.1093/glycob/cwj027. [DOI] [PubMed] [Google Scholar]
- [13].Yang J, Bian W, Gao X, Chen L, Jing N. Nestin expression during mouse eye lens development. Mech. Develop. 2000;94:287–291. doi: 10.1016/s0925-4773(00)00301-4. [DOI] [PubMed] [Google Scholar]
- [14].De iongh RU, Gordon-Thomson C, Chamberlain CG, Hales AM, McAvoy JW. TGF-β receptor expression in lens: Implications for differentiation and cataractogenesis. Exp Eye Res. 2001;72:649–659. doi: 10.1006/exer.2001.1001. [DOI] [PubMed] [Google Scholar]
- [15].Isaka F, Ishibashi M, Taki W, Hashimoto N, Nakanishi S, Kageyama R. Ectopic expression of the bHLH gene Math1 disturbs neural development. Eur J Neurosci. 1999;11:2582–2588. doi: 10.1046/j.1460-9568.1999.00699.x. [DOI] [PubMed] [Google Scholar]
- [16].Hirasawa M, Cho A, Sreenath T, Sauer B, Julien JP, Kulkarni AB. Neuron-specific expression of Cre recombinase during the late phase of brain development. Neurosci Res. 2001;40:125–132. doi: 10.1016/s0168-0102(01)00216-4. [DOI] [PubMed] [Google Scholar]
- [17].Dunker N, Krieglstein K. Reduced programmed cell death in the retina and defects in lens and cornea of Tgfbeta2(-/-) Tgfbeta3(-/-) double-deficient mice. Cell Tissue Res. 2003;31:1–10. doi: 10.1007/s00441-003-0761-x. [DOI] [PubMed] [Google Scholar]
- [18].Iwasaki M, Rayborn ME, Tawara A, Hollyfield JG. Proteoglycans in the mouse interphotoreceptor matrix. V. Distribution at the apical surface of the pigment epithelium before and after retinal separation. Exp Eye Res. 1992;54:415–432. doi: 10.1016/0014-4835(92)90054-v. [DOI] [PubMed] [Google Scholar]
- [19].Lazarus HS, Hageman GS. Xyloside-induced disruption of interphotoreceptor matrix proteoglycans results in retinal detachment. Invest. Ophthalmol. Vis. Sci. 1992;33:364–376. [PubMed] [Google Scholar]
- [20].Burg M, Cole GJ. Characterization of cell-associated proteoglycans synthesized by embryonic neural retinal cells. Arch. Biochem. Biophys. 1990;276:396–404. doi: 10.1016/0003-9861(90)90738-k. [DOI] [PubMed] [Google Scholar]
- [21].Murillo-Lopez F, Politi L, adler R, Hewitt AT. Proteoglycan synthesis in cultures of murine retinal neurons and photoreceptors. Cell Mol. Neurobiol. 1991;11:579–591. doi: 10.1007/BF00741447. [DOI] [PubMed] [Google Scholar]
- [22].Britts PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neural patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- [23].Scott JD. Lens changes in retinal detachment. Trans Ophthalmol Soc U K. 1979;99:241–243. [PubMed] [Google Scholar]
- [24].Brown NP, Bron AJ. Lens disorders: A clinical manual of cataract diagnosis. Butterworth-Heinmann , Jordan Hill; Oxford: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Characterization of TGF-β RI f/f: Nestin-Cre conditional knockout (cKO) mice. PCR analyses of mouse tail DNA for the expression of TGF-β RI floxed (A) and Cre (B) transgenes. LacZ staining of the retinal section from transgenic mice generated by crossing nestin-Cre mice and LacZ reporter mice (C). Immunostaining of retinal sections from wild-type (D, E) and cKO (F, G) mice with anti-neuronal-specific enolase antibody in red (D, F) and anti-TGF-β RI antibody in green (E, G). (Bar=10 μm)