Abstract
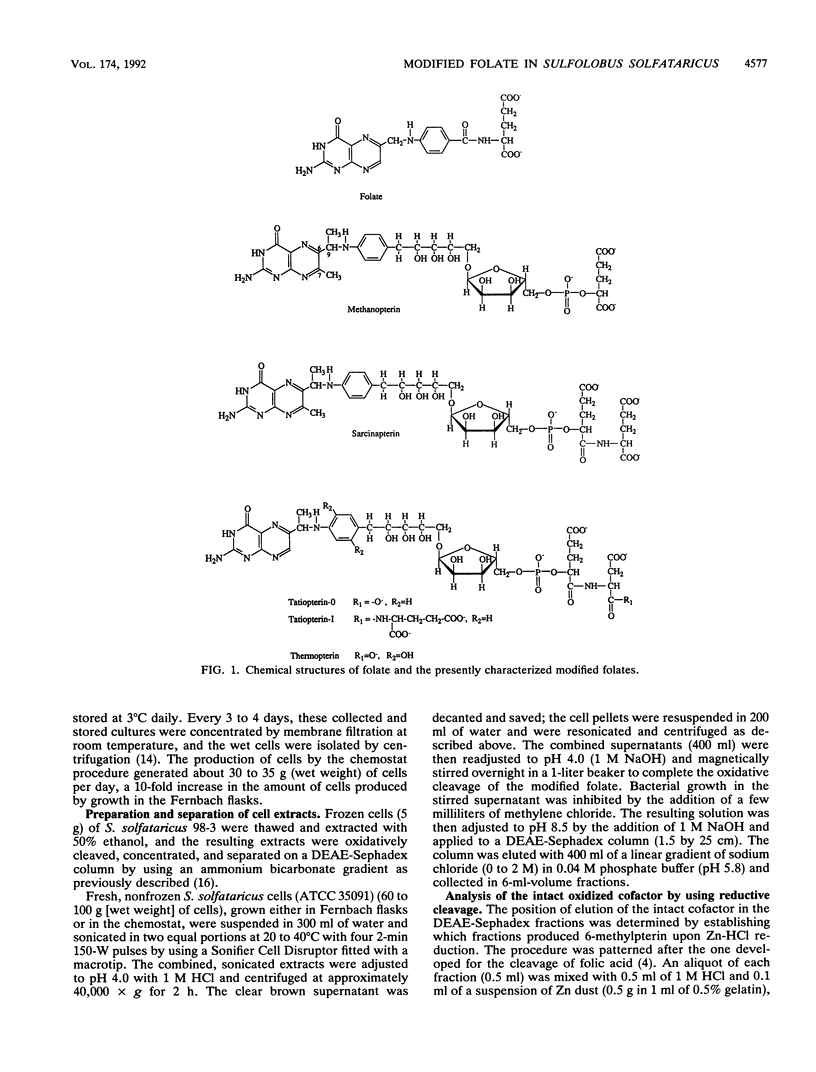
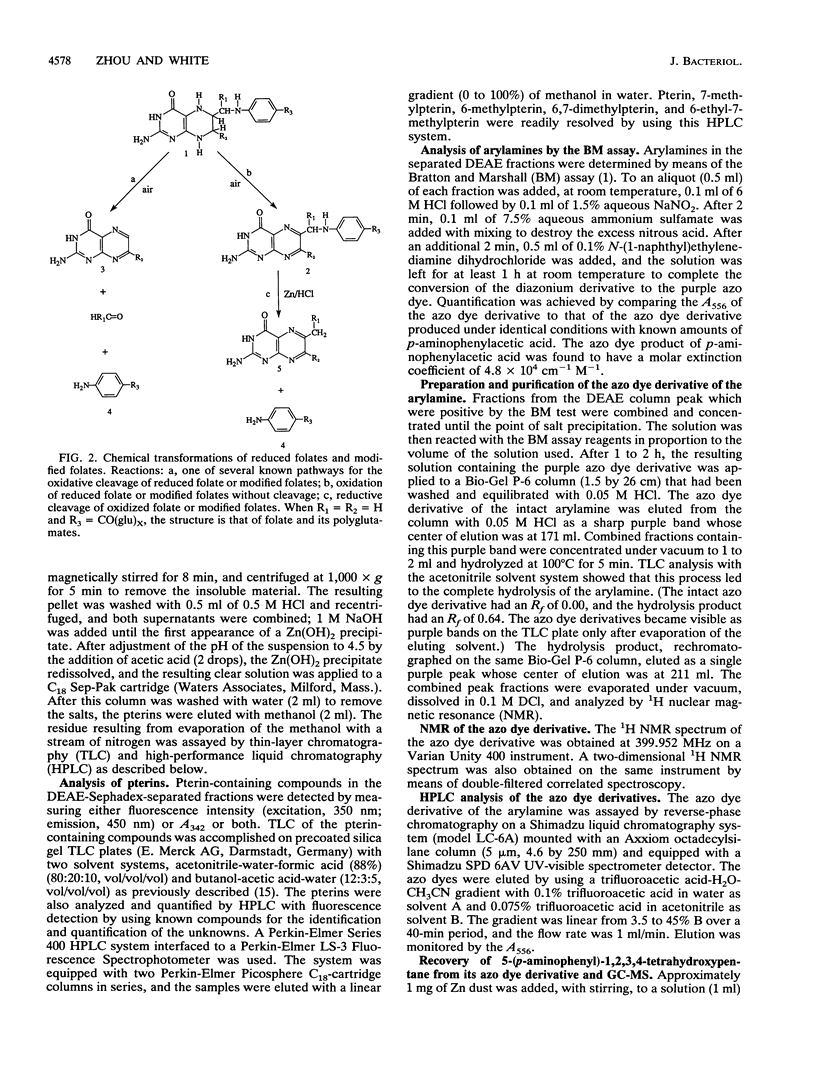
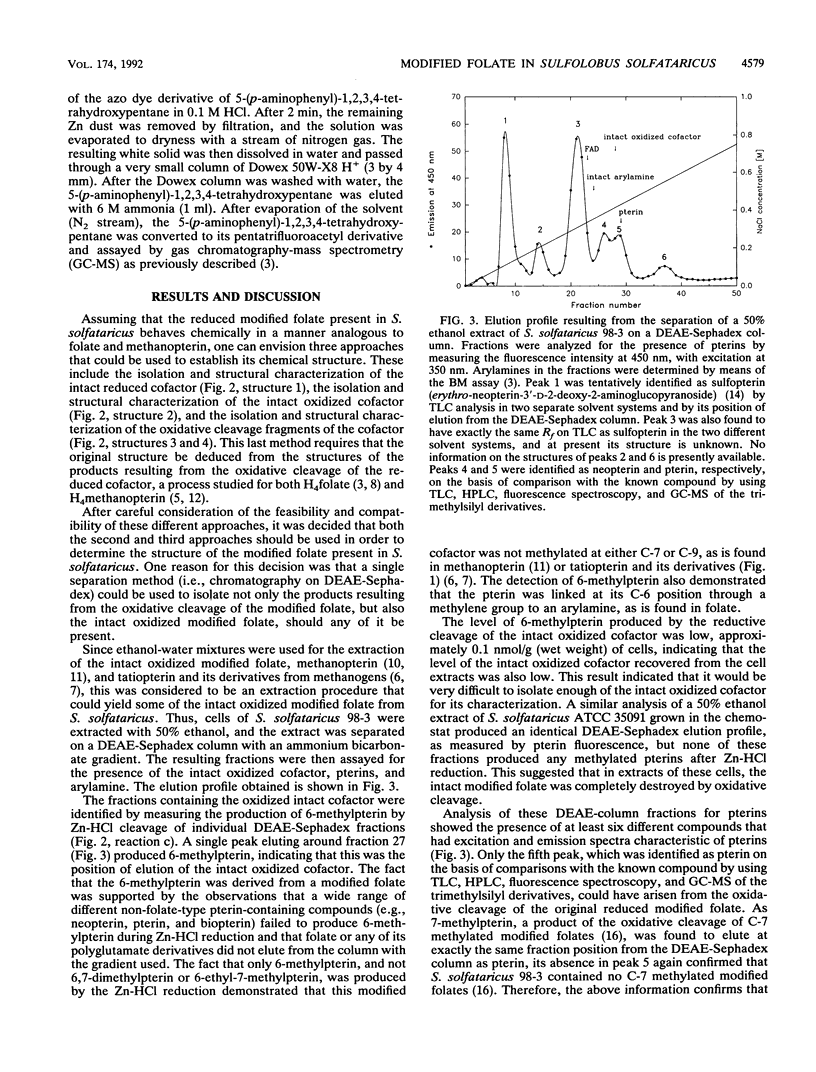
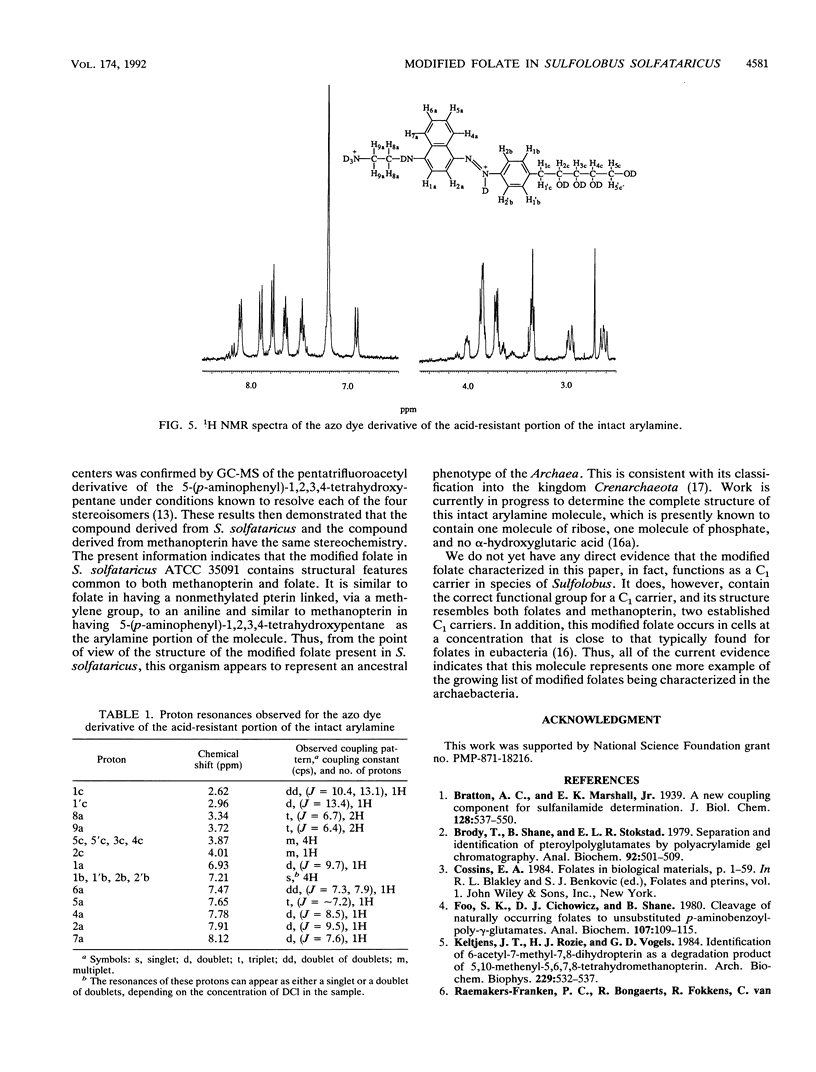
The partial characterization of the modified folate present in Sulfolobus solfataricus has been carried out. Separation of ethanol-water extracts of these cells on a DEAE-Sephadex column led to the isolation of a small amount of intact oxidized cofactor, which, when subjected to reductive cleavage with Zn-HCl, produced 6-methylpterin. This indicated that the modified folate in these cells contained a nonmethylated pterin linked, via a methylene group at the C-6 position of the pterin, to an arylamine, as is found in folate. Oxidative cleavage of intact reduced cofactor produced pterin and a single arylamine. The azo dye derivative of this arylamine was prepared and purified by chromatography on a Bio-Gel P-6 column. The resulting purified compound was shown to be readily hydrolyzed in dilute acid to the azo dye derivative of 5-(p-aminophenyl)-1,2,3,4-tetrahydroxypantane, which was, in turn, readily cleaved to 5-(p-aminophenyl)-1,2,3,4- tetrahydroxypentane by Zn-HCl reduction. The stereochemistry of the resulting 5-(p-aminophenyl)-1,2,3,4-tetrahydroxypentane was shown to be ribo, the same as that of the 5-(p-aminophenyl)-1,2,3,4- tetrahydroxypentane moiety found in methanopterin. The complete arylamine side chain of the modified folate thus contains 5-(p-aminophenyl)-1,2,3,4-tetrahydroxypentane attached, via an acid-labile bond, to a currently unidentified substituent. The modified folate present in S. solfataricus thus contains structural features common to both folates and methanopterin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody T., Shane B., Stokstad E. L. Separation and identification of pteroylpolyglutamates by polyacrylamide gel chromatography. Anal Biochem. 1979 Jan 15;92(2):501–509. doi: 10.1016/0003-2697(79)90691-2. [DOI] [PubMed] [Google Scholar]
- Foo S. K., Cichowicz D. J., Shane B. Cleavage of naturally occurring folates to unsubstituted p-aminobenzoylpoly-gamma-glutamates. Anal Biochem. 1980 Sep 1;107(1):109–115. doi: 10.1016/0003-2697(80)90499-6. [DOI] [PubMed] [Google Scholar]
- Keltjens J. T., Rozie H. J., Vogels G. D. Identification of 6-acetyl-7-methyl-7,8-dihydropterin as a degradation product of 5,10-methenyl-5,6,7,8-tetrahydromethanopterin. Arch Biochem Biophys. 1984 Mar;229(2):532–537. doi: 10.1016/0003-9861(84)90184-x. [DOI] [PubMed] [Google Scholar]
- Lin X. L., White R. H. Structure of solfapterin (erythro-neopterin-3'-D-2-deoxy-2-aminoglucopyranoside) isolated from the thermophilic archaebacterium Sulfolobus solfataricus. J Bacteriol. 1988 Mar;170(3):1396–1398. doi: 10.1128/jb.170.3.1396-1398.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemakers-Franken P. C., Bongaerts R., Fokkens R., van der Drift C., Vogels G. D. Characterization of two pterin derivatives isolated from Methanoculleus thermophilicum. Eur J Biochem. 1991 Sep 15;200(3):783–787. doi: 10.1111/j.1432-1033.1991.tb16245.x. [DOI] [PubMed] [Google Scholar]
- Raemakers-Franken P. C., Voncken F. G., Korteland J., Keltjens J. T., van der Drift C., Vogels G. D. Structural characterization of tatiopterin, a novel pterin isolated from Methanogenium tationis. Biofactors. 1989 Dec;2(2):117–122. [PubMed] [Google Scholar]
- Shane B. Identification of folylpoly(gamma-glutamate) chain length by cleavage to and separation of p-aminobenzoylpoly(gamma-glutamates). Methods Enzymol. 1986;122:323–330. doi: 10.1016/0076-6879(86)22188-6. [DOI] [PubMed] [Google Scholar]
- White R. H. 7-Methylpterin and 7-methyllumizine: oxidative degradation products of 7-methyl-substituted pteridines in methanogenic bacteria. J Bacteriol. 1985 May;162(2):516–520. doi: 10.1128/jb.162.2.516-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. Analysis and characterization of the folates in the nonmethanogenic archaebacteria. J Bacteriol. 1988 Oct;170(10):4608–4612. doi: 10.1128/jb.170.10.4608-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. Distribution of folates and modified folates in extremely thermophilic bacteria. J Bacteriol. 1991 Mar;173(6):1987–1991. doi: 10.1128/jb.173.6.1987-1991.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen P., Labro J. F., Keltjens J. T., Geerts W. J., Vogels G. D., Laarhoven W. H., Guijt W., Haasnoot C. A. Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur J Biochem. 1984 Mar 1;139(2):359–365. doi: 10.1111/j.1432-1033.1984.tb08014.x. [DOI] [PubMed] [Google Scholar]
- van Beelen P., Stassen A. P., Bosch J. W., Vogels G. D., Guijt W., Haasnoot C. A. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984 Feb 1;138(3):563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]


