Etiology and features of osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis (1). Although the precise etiology of the disease in most cases is unknown, it is generally accepted that OA is a multifactorial disorder involving both genetic and environmental components (2). Genetic factors are either mutations or variations in genes that result in defects or variability in cartilage matrix properties and chondrocyte metabolism. In the case of matrix defects caused by mutations in matrix genes, age-dependent cartilage degeneration may occur as a result of even normal mechanical stresses on a joint. In the case of inherited sequence variations in genes, some variations may confer increased risk for OA (3). Environmental factors include obesity, over-loading on joints, repetitive injury involving ligaments and menisci, loss of muscle strength and joint malalignment. These conditions can result in abnormal mechanical stresses on the joint, leading to cartilage degeneration. Since OA can occur as a result of one or a combination of these factors, investigating the detailed pathogenic mechanisms in all these conditions remains a formidable challenge. However, regardless of the nature of the factor(s) that initiate the disease, the pathological progression of OA follows a typical pattern (4). The earliest indication of pathological change is chondrocyte clustering as a result of increased cell proliferation and a general up-regulation of synthetic activity. Increased expression of cartilage-degrading proteinases and matrix proteins suggests an attempt at repair. Gradual loss of proteoglycans appears in the surface region of articular cartilage and this is followed by type II collagen degradation. Cracks develop along the articular surface, producing the histological image termed fibrillation. At later stages of the disease, fibrocartilage forms, probably as a consequence of unsuccessful attempts by chondrocytes to fill in the cracks. Finally, osteophytes, bony structures at the periphery of the joint surface, form.
The similarities in the pathologic progression of the disease, even when the initiating events are different, indicate that there may be a common molecular sequence of events underlying OA progression. Thus, studies of pathogenetic changes, even in cases of more rare forms of OA, are likely to provide significant information about disease mechanisms and therapeutic targets for treating even more common forms of the disease. During the past few years, mutations in genes encoding types II, IX and XI collagens have been demonstrated to be associated with an age-dependent articular cartilage degenerative process that has all the histological hallmarks of OA (5-15). These genetic forms of OA in humans clearly represent rare genetic subsets of the disease, but studies of these genetic forms are likely to provide insights into the pathogenesis of more common forms as well.
OA associated with mutations in types IX and XI collagens in humans: Multiple epiphyseal dysplasia (MED) and Stickler syndrome
MED, a heterogeneous group of hereditary chondrodysplasias characterized by early onset OA and mild short stature in humans, is caused by mutations in type IX collagen (COL9A1, COL9A2, and COL9A3), cartilage oligomeric matrix protein (COMP), or matrilin 3 (16,17). Stickler syndrome (18) is a relatively mild form of osteochondrodysplasia caused by mutations in the genes encoding type XI collagen chains (COL11A1 and COL11A2) or type II collagen (COL2A1). Types II, IX and XI collagens form cartilage collagen fibrils (19, 20) that offer high tensile strength and promote the retention of the highly hydrated proteoglycans in articular cartilage. A defect in the structural components in the matrix, such as mutations in type IX collagen (MED) or type XI collagen (Stickler syndrome) result in disorganization of the collagen network and makes the articular cartilage less resistant to the normal mechanical loading of the joint surface. Of clinical interest is that OA in MED patients appears mostly in knee joints, with the hip joint often spared from the degenerative process. In contrast, patients with Stickler syndrome have OA in hips as well as knees. Why knee joints are primarily “targeted” in MED patients is unknown. One plausible explanation is that the shape of a joint, particularly a knee joint, is a critical element in the maintenance of normal joint function. An alteration of the joint surface, as seen in knee joints of MED patients with type IX collagen mutations, will unevenly allocate the mechanical stresses on the joint. Therefore, OA in knee joints of MED patients with type IX collagen mutations may be the result of a combined effect of altered joint geometry and a defective matrix.
Mouse models for genetic forms of human OA, type IX collagen-deficient (Col9a1-/-) and type XI collagen-deficient (cho/+) mice
Pathological changes in human OA are complex and occur over a relatively long period of time in affected individuals. Hence, an appropriate mouse OA model must show a pathological alteration in joints similar to what is seen in humans with the changes developing over time. Both Col9a1-/- and cho/+ mice possess those features of human OA. Col9a1-/- mice were generated by gene-targeting (21). The deletion of Col9a1 leads to the absence of type IX collagen (22). Col9a1-/- mice develop normally with the exception of a flattening of knee joint surfaces and an increased distance between the knee joint condyles. The chondrodysplasia in the cho mouse strain is an autosomal-recessive disorder (23). We previously found that the defect in homozygous cho/cho mice, which die at birth, is due to a single nucleotide deletion in Col11a1 leading to a frame-shift and premature termination of translation of the α1 chain of type XI collagen and that the level of mRNA encoded by the mutant Col11a1 is dramatically reduced (24). Heterozygous cho/+ mice develop normally without obvious skeletal abnormalities at birth. Histological studies show that Col9a1-/- and cho/+ mice develop OA-like changes in knee and temporomandibular (TM) joints starting at the age of 3 months and a severe OA-like pathology over 9 to 12 months (normal life span about 30 months on average) (25,26). The OA-like phenotype in Col9a1-/- mice is a good model for the cartilage degenerative condition seen in MED patients with type IX collagen mutations; the cho/+ mice serve as a model for the degenerative cartilage condition seen in Stickler syndrome caused by type XI collagen mutations. Importantly, we found that the expression of matrix metalloproteinase 13 (Mmp-13) is increased in the articular cartilage of knee and TM joints in both Col9a1-/- and cho/+ mice at the age of 6 months. The level of Mmp-derived type II collagen fragments is also increased in both mutants, indicating that Mmp activity is elevated as well. Interestingly, we have found that the expression of discoidin domain receptor 2 (Ddr2), a cell surface type II collagen receptor, is also increased in mutant mice at a time when the expression of Mmp-13 is upregulated.
Metalloproteinase 13 (MMP-13) in OA
A study by Verzijl et. al. (27) indicate that the half-life of cartilage collagen is 117 years. This suggests that chondrocytes may have a limited ability to produce type II collagen in mature articular cartilage once the collagen is degraded. Thus, type II collagen breakdown in articular cartilage may be a “rate-determining” step in OA progression. Identification of a drug that can prevent type II collagen degradation may be able to prolong the progression of OA and delay the need for joint replacement. Recent studies suggest that MMP-13 (collagenase-3) is particularly important in OA progression because of its ability to cleave triple-helical type II collagen more efficiently than MMP-1 (collagenase-1) (28). MMP-13 cleaves the chains of type II collagen at a Gly906-Leu907 site to generate two fragments corresponding to 3/4 and 1/4 of the intact molecule. In addition, the enzyme can further cleave at a secondary site, Gly909-Gln910, within the smaller (1/4) fragment. MMP-13 is expressed at a very low level in normal articular cartilage, but the expression of the enzyme is increased in human OA articular cartilage (29-32). This is consistent with the observation that constitutive expression of MMP-13 in mouse cartilage results in OA-like changes in knee joints (33). Transcriptional analysis of the MMP-13 shows that the human MMP-13 promoter contains a TATA box and binding sites for transcription factors such as AP-1 (e.g. c-Fos/c-Jun), and PEA-3 and Runx2. Mutations in these binding sites can partially or completely abolish the activity of the human MMP-13 promoter (34-38). Several signaling pathways involved in the transcriptional regulation of MMP-13 expression in response to the stimulation by IL-1β and fibronectin fragments (FN-f) have been characterized in chondrocytes (39-44). IL-1β binding to its receptor results in signaling through the JNK and p38 kinase cascades, but not ERK1/2, and can also activate the NF-κB pathway. The integrin α5β1 is a receptor for FN-f. After binding to FN-f, the receptor is activated and interacts with proline-rich tyrosine kinase-2 (PYK2) and the focal adhesion kinase (FAK) to form a signaling complex. This complex can activate ERK, JNK and p38. Activation of the p38 pathway increases the transcription of Runx2, whereas activation of ERK results in phosphorylation of Runx2 protein in the cytoplasm and subsequent translocation to the nucleus. Although the signaling pathways activated by the integrins and cytokine receptors on chondrocytes play a significant role in the transcriptional regulation of MMP-13 expression in chondrocytes, a certain degree of matrix damage would be required to generate the FN-f, which induce the resident chondrocytes to synthesize and release MMP-13 through the integrin-mediated pathways.
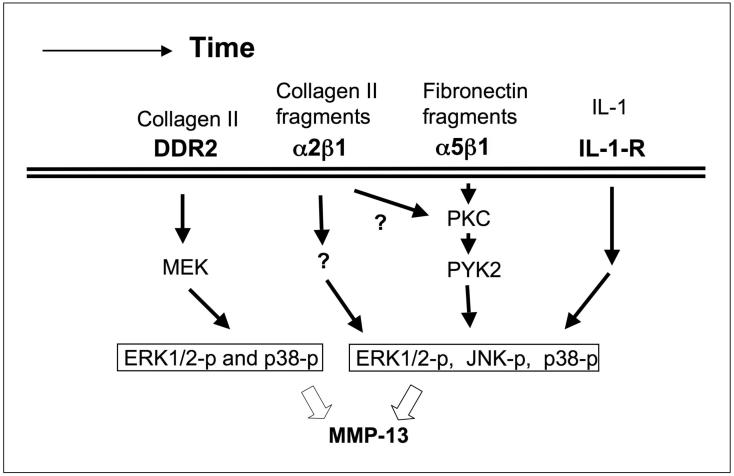
One important question is whether any mechanism exists at an early stage of OA to stimulate chondrocytes to synthesize and release MMP-13 prior to significant degradation of the cartilage. The results of our studies and those of others suggest that DDR2 may provide such a mechanism on chondrocytes (Figure 1).
Figure 1.
Diagram showing a proposed timeline for the progression of OA. At an early stage, activation of the collagen receptor DDR2 leads to upregulated expression of MMP13. This results in generation of fragments of collagen II and fibronectin in the territorial matrix. These fragments, as well as cytokines such as IL-1, further stimulate signaling pathways that induce MMP-13 expression. The result is a time-dependent increase in the degradation of the cartilage extracellular matrix.
Role of discoidin domain receptor 2 (DDR2) in the regulation of MMP-13 expression
Discoidin domain receptors 1 and 2 (DDR1 and DDR2) were originally cloned as cell surface receptor tyrosine kinases (RTKs) (45-48). The mRNA transcripts of the receptors are detected in several human and mouse tissues, but mainly in bone, cartilage, skin, skeletal muscle, brain and heart. We examined the expression of DDR1 and DDR2 in mouse articular cartilage. We found that DDR2 is expressed at a very low level in the superficial layer of normal articular cartilage, whereas the expression of DDR1 is hardly detectable. In 1997, two research groups reported that native collagens were the DDR ligands (49,50) and that a synthetic short triple-helical collagen-like (10 repeats of Gly-Pro-Hyp) peptide is unable to activate DDRs. A recent study demonstrates that a region of 234 amino acid residues within type II collagen molecules contains a specific binding site for DDR2, that type II collagen is recognized preferentially by DDR2 and that the N-terminal discoidin homology (DS) domain of DDR2 is required for the interaction of DDR2 with type II collagen (51). Ddr2-deficient mice were generated by gene targeting techniques and mutant mice exhibit short stature probably due to decreased proliferation of chondrocytes in growth plates (52). One intriguing finding is that over-expression of DDR2 in fibroblast-like cell lines enhances the expression of MMP-1. This prompted us to investigate if DDR2 controls the synthesis of matrix-degrading enzymes in chondrocytes.
The results from our in vivo studies demonstrate that the expression of DDR2 and MMP-13 and the level of MMP-derived type II collagen fragments are increased in articular cartilage of knee and TM joints in Col9a1-/- and cho/+ mice (25,26,53). We also have found that the expression of DDR2 and MMP-13 and the level of MMP-derived type II collagen fragments are elevated in the articular cartilage of mice in which OA is induced surgically and in articular cartilage of human OA hip joints obtained from joint replacement surgeries (54). This indicates that the increased expression of these genes is not unique to cases of genetic OA caused by mutations in cartilage collagens, but is also seen in cases where the initiating event is joint instability or some other non-genetic event. To understand whether or not the increased expression of DDR2 and MMP-13 in articular cartilage are independent events, we performed a series of in vitro experiments (55). Results from these experiments demonstrate that 1) the level of MMP-13 mRNA is elevated in human chondrocytes and mouse primary chondrocytes cultured on native type II collagen for 24 h. Surprisingly, we found that the level of DDR2 mRNA is also increased in human and mouse chondrocytes cultured on native type II collagen. This indicates that chondrocytes exposed to native type II collagen for 24 h are induced to express increased levels of DDR2 and MMP-13 mRNA. In contrast, the mRNAs levels of MMP-1, MMP-3, MMP-8, ADAMTS-4, ADAMTS-5 and IL-1 were not increased in the cells. 2) When human chondrocytes were cultured on denatured type II collagen (gelatin), the levels of expression of MMP13 and DDR2 mRNA were not elevated, suggesting that chondrocytes respond in a specific manner to triple-helical type II collagen. 3) The overexpression of full-length DDR2 cDNA results in increased level of MMP-13 mRNA and overexpression of a truncated DDR2 cDNA (lacking the protein tyrosine kinase of the cytoplasmic domain of the receptor) decreases the expression of MMP-13 mRNA in cells cultured on type II collagen for 24 h. In addition, DDR2 lacking the type II collagen binding domain (discoidin domain) of the receptor has no effect on the expression of MMP-13 mRNA and receptor itself, indicating that the interaction of chondrocytes with type II collagen is required for the increased MMP-13 mRNA levels in chondrocytes. 4) Ras/Raf/MEK/ERK and p38 signaling pathways are involved in the increased expression of MMP-13 in chondrocytes by type II collagen-DDR2 interaction. 5) IL-1β is not an intermediate in the DDR2 signaling pathway involved in the up-regulation of MMP-13 in chondrocytes. 6) DDR2 and integrin α5β1 signaling pathways have a synergistic effect on the expression of MMP-13 in chondrocytes, suggesting that DDR2 signaling is independent of α5β1 signaling. 7) DDR2 signaling increases the transcriptional activity of the MMP-13 promoter in chondrocytes. Based on these data, we conclude that the activation of DDR2 by type II collagen increases the expression of MMP-13 via ERK and p38 signaling pathways in chondrocytes.
A pathogenetic mechanism for OA in Col9a1-/- and cho/+ mice
We hypothesize that once the collagen-proteoglycan network in the extracellular matrix of cartilage is disrupted/weakened, chondrocytes become more vulnerable to mechanical stress. Under these conditions, even normal mechanical loads can activate chondrocytes. Thus, we observe increased chondrocyte activities at early stages of OA in mice, including chondrocyte clustering (proliferation) and increased synthesis of proteoglycans. Over time, the activated chondrocytes synthesize and release matrix-degrading enzymes, which degrade proteoglycans. One of the consequences of proteoglycan degradation is enhanced exposure of chondrocytes to type II collagen fibrils. Type II collagen fibrils are present within territorial and interterritorial locations in normal articular cartilage, with little or no type II collagen close to the chondrocyte surface (56). Interaction of chondrocytes with type II collagen fibrils may result in enhanced signaling through DDR2. The activation of DDR2 induces the expression of MMP-13 as well as expression of DDR2 itself. MMP-13 degradation of type II collagen results in type II collagen fragments (CII-f), which in turn may bind to integrins such as α2β1 to activate signals that further increase the synthesis of MMP-13 (Figure 1). The result is a feedback amplification loop that enhances the degradation of articular cartilage. The end result is the irreversible destruction of articular cartilage. Thus, type II collagen in close contact with the cell surface can regulate its own proteolysis via its cell membrane receptor.
Although our data demonstrate that type II collagen-induced DDR2 increases the expression of MMP-13 in chondrocytes by activating ERK and p38 pathways, we do not believe that ERK and p38 are appropriate therapeutic targets for slowing OA progression, since the signaling pathways in which they participate have significant biological roles in many different cell types. A better target may be a common chondrocyte-specific down-stream effector of ERK and p38, such as one of the mitogen kinases (MKs). In addition, development of a chondrocyte-specific antagonist of DDR2 may also be of interest in the effort to develop novel therapies for OA.
Acknowledgements
The studies reviewed here were support by NIH grants AR36819 (to BRO) and AR51989 and AR050245 (to YL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Peyron JG, Altman RD. The epidemiology of osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, editors. Osteoarthritis. 1992. pp. 15–37. [Google Scholar]
- 3.Reginato AM, Olsen BR. The role of structural genes in the pathogenesis of osteoarthritic disorders. Arthritis Res. 2002;4:337–45. doi: 10.1186/ar595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamerman D. The biology of osteoarthritis. The New England J Med. 1989;320:1322–30. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- 5.Knowlton RG, Katzenstein PL, Moskowitz RW, Weaver EJ, Malemud CJ, Pathria MN, et al. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990;322:526–30. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- 6.Vikkula M, Palotie A, Ritvaniemi P, Ott J, Ala-Kokko L, Sievers U, et al. Aho K, Peltonen L. Early-onset osteoarthritis linked to the type II procollagen gene. Detailed clinical phenotype and further analyses of the gene. Arthritis Rheum. 1993;36:401–9. doi: 10.1002/art.1780360317. [DOI] [PubMed] [Google Scholar]
- 7.Ritvaniemi P, Korkko J, Bonaventure J, Vikkula M, Hyland J, Paassilta P, et al. Identification of COL2A1 gene mutations in patients with chondrodysplasias and familial osteoarthritis. Arthritis Rheum. 1995;38:999–1004. doi: 10.1002/art.1780380717. [DOI] [PubMed] [Google Scholar]
- 8.Mustafa Z, Chapman K, Irven C, Carr AJ, Clipsham K, Chitnavis J, et al. Linkage analysis of candidate genes as susceptibility loci for osteoarthritis-suggestive linkage of COL9A1 to female hip osteoarthritis. Rheumatology. 2000;39:299–306. doi: 10.1093/rheumatology/39.3.299. [DOI] [PubMed] [Google Scholar]
- 9.Czarny-Ratajczak M, Lohiniva J, Rogala P, Kozlowski K, Perala M, Carter L, et al. A mutation in COL9A1 causes multiple epiphyseal dysplasia: further evidence for locus heterogeneity. Am J Hum Genet. 2001;69(5):969–80. doi: 10.1086/324023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muragaki Y, Mariman EC, van Beersum SE, Perala M, van Mourik JB, Warman ML, et al. A mutation in the gene encoding the alpha 2 chain of the fibril-associated collagen IX, COL9A2, causes multiple epiphyseal dysplasia (EDM2) Nat Genet. 1996;12:103–5. doi: 10.1038/ng0196-103. [DOI] [PubMed] [Google Scholar]
- 11.Bonnemann CG, Cox GF, Shapiro F, Wu JJ, Feener CA, Thompson TG, et al. A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. PNAS. 2000;97:1212–17. doi: 10.1073/pnas.97.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger J. The type XI collagenopathies. Pediatr Radiol. 1998;28:745–50. doi: 10.1007/s002470050459. [DOI] [PubMed] [Google Scholar]
- 13.Sirko-Osadsa DA, Murray MA, Scott JA, Lavery MA, Warman ML, Robin NH. Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha2(XI) chain of type XI collagen. J Pediatr. 1998;132:368–71. doi: 10.1016/s0022-3476(98)70466-4. [DOI] [PubMed] [Google Scholar]
- 14.Richards AJ, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Hum Mol Genet. 1996;5:1339–43. doi: 10.1093/hmg/5.9.1339. [DOI] [PubMed] [Google Scholar]
- 15.Vikkula M, Mariman EC, Lui VC, Zhidkova NI, Tiller GE, Goldring MB, et al. Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell. 1995;80:431–7. doi: 10.1016/0092-8674(95)90493-x. [DOI] [PubMed] [Google Scholar]
- 16.Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FFB, Harrison WR, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–9. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 17.Borochowitz ZU, Scheffer D, Adir V, Dagoneau N, Munnich A, Cormier-Daire V. Spondylo-epimetaphyseal dysplasia (SEMD) matrilin 3 type: homozygote matrilin 3 mutation in a novel form of SEMD. J Med Genet. 2004;41:366–72. doi: 10.1136/jmg.2003.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snead MP, Yates JR. Clinical and Molecular genetics of Stickler syndrome. J Med Genet. 1999;36:353–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Eyre DR, Wu J. Collagen structure and cartilage matrix integrity. J Rheum. 1995;22:83–5. [PubMed] [Google Scholar]
- 20.Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: type XI collagen is restricted to thin fibrils. J Histochem Cytochem. 1995;43:967–79. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- 21.Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, et al. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. PNAS. 1994;91:5070–74. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagg R, Hedbom E, Mollers U, Aszodi A, Fassler R, Bruckner P. Absence of the alpha1(IX) chain leads to a functional knock-out of the entire collagen IX protein in mice. J Biol Chem. 1997;272:20650–54. doi: 10.1074/jbc.272.33.20650. [DOI] [PubMed] [Google Scholar]
- 23.Seegmiller R, Fraser FC, Sheldon H. A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis. J Cell Biol. 1971;48:580–93. doi: 10.1083/jcb.48.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lacerda DL, Warman ML, Beier DR, Oxford JT, Morris NP, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–30. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48(9):2509–18. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- 26.Hu K, Xu L, Cao L, Flahiff CM, Setton LA, Youn I, et al. Pathogenesis of Osteoarthritis-like Changes in Joints of Type IX Collagen-Deficient Mice. Arthritis. Rheum. 2006;9:2891–900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 27.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of Collagen Turnover on the Accumulation of Advanced Glycation End Products. J Biol Chem. 2000;275:39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 30.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–57. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 31.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–19. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J C Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269(24):16766–73. [PubMed] [Google Scholar]
- 35.Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40(2):222–33. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 36.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;8-9:519–26. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 37.Porte D, Tuckermann J, Becker M, Baumann B, Teurich S, Higgins T, et al. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene. 1999;18(3):667–78. doi: 10.1038/sj.onc.1202333. [DOI] [PubMed] [Google Scholar]
- 38.Hess J, Porte D, Munz C, Angel P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem. 2001;276:20029–38. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- 39.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–11. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Mengshol JA, Vincenti MP, Brinckerhoff CE. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001;29:4361–72. doi: 10.1093/nar/29.21.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–76. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 42.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–62. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 43.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–64. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278(27):24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8:2731–39. [PubMed] [Google Scholar]
- 46.Di Marco, Cutuli E, Guerra N, Cancedda L, De Luca M. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J Biol Chem. 1993;268:24290–95. [PubMed] [Google Scholar]
- 47.Lai C, Gore M, Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9:2567–78. [PubMed] [Google Scholar]
- 48.Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, et al. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993;8:3433–40. [PubMed] [Google Scholar]
- 49.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Molecular Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 50.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Molecular Cell. 1997;1(1):25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 51.Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J. Mol. Biol. 2004;344:993–03. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 52.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2:446–52. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, et al. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Archives Oral Biol. doi: 10.1016/j.archoralbio.2006.10.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Peng H, Glosson S, Lee PL, Hu H, Ijiri K, et al. Increased Expression of a Collagen Receptor, Discoidin Domain Receptor 2, in Articular Cartilage as a Common Event in the Pathogenesis of Osteoarthritis. Arthritis Rheum. doi: 10.1002/art.22761. (in press) [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–55. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 56.Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37:271–84. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]