Abstract
Objective
To determine the effects of a thiazolidinedione (TZD) agonist of peroxisome proliferator-activated receptor (PPAR)-γ, rosiglitazone, in a baboon model of established endometriosis.
Design
Prospective, randomized, placebo-controlled study.
Setting
Experimental surgery laboratory at the Institute of Primate Research in Nairobi, Kenya.
Animal(s)
Endometriosis was induced using intrapelvic injection of eutopic menstrual endometrium in 12 female baboons with a normal pelvis that had undergone at least one menstrual cycle since the time of captivity.
Intervention(s)
Induction of endometriosis by laparoscopy was performed in 12 baboons with a normal pelvis. Endometrial tissue was extracted from each baboon by curettage and a standard amount of endometrium was then seeded onto several peritoneal sites as previously described. About 34-68 days after the induction of laparoscopy, a pre-treatment laparoscopy (baseline disease assessment) was performed in the baboons to record the extent of endometriotic lesions. The 12 baboons were randomized into 3 groups and treated from the day after the staging laparoscopy for a total duration of 30 days. They received either PBS tablets (n=4, placebo control; placebo tablets once a day by mouth for 30 days), GnRH-antagonists (n=4, active control; Ganirelix acetate 125 μg/day for 30 days) or rosiglitazone (n=4, test drug, 2 mg by mouth each day for 30 days). A 3rd and final laparoscopy on day 30 after the start of treatment was performed to record the extent of endometriosis. The type of lesion (typical, red, white and suspicious) was recorded. Biopsies were obtained to confirm the histological presence of endometriosis.
Main Outcome Measure(s)
A videolaparoscopy was performed 30 days after treatment to document the number and surface area of endometriotic lesions as well as to calculate the revised American Society for Reproductive Medicine score (rAFS) and stage.
Result(s)
The surface area of endometriotic lesions was statistically significantly lower in rosiglitazone treated baboons when compared to the placebo group (P<0.05). Baboons treated with rosiglitazone or Ganirelix had a greater negative relative change in surface area of peritoneal endometriotic lesions than controls (P<0.05). The overall, weighted appearance of the lesion types suggests that rosiglitazone may deter the development of newer endometriotic lesions.
Conclusion(s)
A PPAR-γ ligand, rosiglitazone, can effectively diminish the burden of endometriosis disease in the baboon endometriosis model. This animal model holds promise that a TZD drug may be helpful in women with endometriosis.
Keywords: baboon, endometriosis, immunosuppression, PPAR-γ, rosiglitazone, thiazolidinedione
Introduction
Endometrial tissue (endometrial glands and stroma) found outside of the uterus defines endometriosis. The prevalence in women with pelvic pain and subfertility, 60%, is much greater than that seen for the reproductive age group in general, 7-15% (1). Disease severity based on visual scoring has poor correlation with either pelvic pain or subfertility (2, 3). Nevertheless, this disease, at any stage, is a common etiology for women who present with chronic pelvic pain while still desiring to conceive. At this time, effective treatment concurrently addressing both arms is lacking.
There is a need for an optimal drug that could allow for both pain management and continued attempts at conceiving. However, development of more efficacious, better-tolerated and more affordable treatment options has been slow. An ideal treatment would eliminate endometriotic lesions, prevent recurrence and not impede ovulation. Immune modulating drugs have been studied as candidate treatment options (4-8) including a recent successful study of recombinant human tumor necrosis factor binding protein in the baboon model of endometriosis (9-11).
Peroxisome proliferator-activated receptors (PPARs) are a new class of immune modulators (12-14) found in adipose tissue, liver, spleen, colon, adrenal gland, muscle tissue, macrophages and endometrial epithelial and stromal cells (15-17). PPAR-γ ligands have been shown to decrease aromatase activity in cultured human granulosa cells (18). We published our study using a PPAR-γ agonist, ciglitazone, in the rat model of endometriosis to evaluate a new approach to immunomodulation of endometriosis (19). The uterine autograft from ciglitazone-treated rats showed marked epithelial changes and overall regression of the explant. The post-treatment spherical volume, weight and epithelial score was significantly lower in ciglitazone treated groups than in controls.
Surgically transplanted endometrial tissue in the baboon provides a primate model to study the effects of experimental drugs on ectopic endometrial tissue (10, 11, 20). Baboon endometriotic implants show histologic transformations similar to those seen in human endometriotic lesions. In the current study, we proposed that an oral PPAR-γ agonist, rosiglitazone, could suppress the immune response as well as diminish estrogen production from endometriotic lesions and lead to a reduction in the number of existing lesions. Therefore, the objective of this study was to assess the ability of rosiglitazone in the baboon model of endometriosis to test if rosiglitazone could impede the growth of established ectopic endometrial tissue.
Materials and Methods
Animals and laparoscopy
Twelve female baboons (Papio anubis) of proven fertility (10-17 kg) were studied at the Institute of Primate Research. All animals were tested and only those that were negative for common pathogens (bacterial and viral infections and parasites) were used in this study. Prior to study initiation, each animal had undergone at least one menstrual cycle in captivity. Animals were housed in single cages. The baboons were randomly selected for treatment arm just prior to the staging laparoscopy (laparoscopy 2) until there were four baboons per treatment group, 12 overall. All animal procedures and care were conducted in accordance with the Institute of Primate Research standard operating procedures. The Institutional Scientific Evaluation and Review Committee (ISERC) and Animal Care and Use Committee (ACUC) of the Institute of Primate Research approved the study.
Induction of endometriosis
On the first or second day after onset of menses, endometrial tissue was extracted from each baboon by uterine curettage and fragmented through an 18-gauge needle. During laparoscopy, the resulting paste (1000 ± 250 mg) was autologously seeded onto various peritoneal sites (uterosacral ligaments, uterovesical fold, pouch of Douglas, ovaries and ovarian fossae), as described previously (20). A second videolaparoscopy was performed 52.3 ± 12.3 days (mean ± SD; range, 34-68 days) later to document the number, surface area and volume of the endometriotic lesions, to determine the presence, localization and extent of adhesions, and to calculate the adapted rAFS score and assigned stage of disease according to the revised classification system of the American Society for Reproductive Medicine (21). Other adhesions that were not related to the ovary, Fallopian tube and cul-de-sac and that were observed between individual peritoneal endometriotic lesions and pelvic organs were recorded separately. The surface area (mm2) of an endometriotic lesion (and an endometriotic lesion-related adhesion) was determined by multiplying length (mm) x width (mm). The total cumulative surface area and total cumulative number of lesions were calculated for each baboon. At least one biopsy of an endometriotic lesion was taken from each baboon for pathologic confirmation of the disease.
For each baboon, changes in the pattern of the menstrual and the peritoneal cycle were carefully monitored during the study. In baboons, perineal inflation and deflation correspond with follicular and luteal phase, respectively. Ovulation is known to occur approximately 3 days before perineal deflation, with a margin of error of 2 days (22). Daily perineal inspection in each baboon allowed determination of the onset of perineal inflation (start of the perineal cycle, corresponding to the initiation of the follicular phase) and perineal deflation during the total duration of the study period.
Blood samples for determination of estradiol and progesterone were obtained in each baboon at the time of the staging laparoscopy and again at the final day of treatment laparoscopy, 30 days later.
Histology
Biopsies were formalin fixed and embedded in paraffin blocks, sectioned at 5 μm thickness, stained with hematoxylin and eosin, and examined using a light microscope. Histological confirmation of the clinical diagnosis of endometriosis was defined as the presence of both endometrial glands and stroma in biopsies of suspected endometriotic lesions.
Drug treatment
The PPAR-γ agonist, rosiglitazone used in this study was manufactured by GlaxoSmithKline (Avandia, Research Triangle Park, NC). A GnRH antagonist, Ganirelix (Organon, Roseland, NJ), was used as an active comparator. A placebo tablet was provided by the University of Michigan Investigational Drug Services. Each baboon was given 2 mg/day of rosiglitazone. The mean baboon weight at time of treatment was 13.3 kg. An equivalent dose in a typical 65 kg woman would be 9.92 mg/day of rosiglitazone. The current FDA-approved dose of rosiglitazone is a maximum of 8 mg daily. Due to uncertain absorption from oral administration of rosiglitazone hidden in a banana, the dose appears within the FDA-approved range of 4-8 mg daily. A Ganirelix dose was determined from prior suppressive doses utilized in similar baboons at the Institute of Primate Research.
12 baboons were randomly assigned to treatment with either placebo tablet (n=4; tablet orally daily), Rosiglitazone (n=4; 2 mg tablet orally daily) or GnRH antagonist (n=4; Ganirelix 125 μg sc daily). One-half the recommended human dose of Ganirelix was utilized despite the baboon weighing roughly one-fifth an average woman because the resorption of sc drugs in baboons can be variable. All subcutaneous injections were given during a 10 minute period of general anesthesia, induced by an IM injection of 1 mg/kg/ketamine (Ketamin, Sanofi) and 0.5 mg/kg xylazine (Xylalin, Apharmo).
Hormone assays
Both serum estradiol and progesterone levels were analyzed on a fully automated competitive direct chemiluminescence immunoassay (Bayer-ADVIA Centaur, Tarrytown, NY), using a heterogeneous competitive magnetic separation assay. The analytical sensitivity of the estradiol assay was 10 pg/mL for estradiol and 0.21 ng/mL for progesterone. The functional sensitivity of the estradiol assay was 38.2 pg/mL; functional sensitivity being defined as the analyte concentration in serum at which total imprecision of 20% coefficient of variation (CV) is observed. The within-run CV was <13% for both the estradiol and progesterone assays.
Statistical analysis
The data were analyzed using univariate ANOVA followed by post hoc analyses with a Bonferroni test (SPSS 14.0.2; Chicago, IL). The level of significance was taken as P<0.05. Values are expressed as median ± SD. A relative change value was calculated for the surface areas by the following equation: Surface area after treatment (laparoscopy 3) minus surface area before (laparoscopy 2) divided by surface area before (laparoscopy 2).
Results
Endometriosis
The presence of endometriosis was histologically confirmed in all baboons. The number, rAFS score and stage of disease along with the number of adhesions were comparable in all three groups and are shown in Table 1 before and after the 30 days of respective treatment. Table 2 lists the change in number of typical and red lesions among all three treatment groups before and after the treatment month. Representative macroscopic appearances of the endometriotic lesions in the three treatment groups are shown in Figures 1A-C.
Table 1.
Overall results for pre-and post-treatment.
| Placebo | GnRH-antagonist | Rosiglitazone | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| n | 4 | 4 | 4 | 4 | 4 | 4 |
| Total SA (mm2) (+ adhesions) | 145, 219, 314, 45 (180.8 ± 113.9) | 162, 209, 235, 53 (164.6 ± 80.6) | 191, 459, 430 180 (315 ± 150.1) | 109, 118, 389, 96 (177.9 ± 141.2) | 209, 328, 134, 291 (240.3 ± 86.8) | 55, 235, 87, 116 (123.2 ± 78.3) |
| Total SA (mm2) (- adhesions) | 65, 195, 180, 45 (121.3 ± 77.2) | 32, 193, 74, 53 (89.6 ± 71.8) | 133, 94, 114, 98 (109.8 ± 17.7) | 50, 64, 60, 50 (55.9 ± 7.3) | 209, 83, 59, 75 (106.3 ± 69.2) | 55, 45, 29, 40 (42.2 ± 10.8) |
| No. total lesions | 8, 13, 7, 10 (9.5 ± 2.6) | 7, 13, 6, 11 (9.3 ± 3.3) | 9, 9, 8, 15 (10.3 ± 3.2) | 9, 9, 11, 14 (10.8 ± 2.4) | 15, 18, 7, 13 (13.3 ± 4.6) | 15, 16, 6, 13 (12.5 ± 4.5) |
| Total no. red lesions | 4, 3, 0, 0 (1.8 ± 2.1) | 3, 6, 0, 3 (3± 2.4) | 4, 1, 1, 11 (4.3± 4.7) | 1, 2, 2, 5 (2.5 ± 1.7) | 6, 6, 5, 0 (4.3 ± 2.9) | 1, 0, 2, 0 (0.8 ± 1, 3) |
| Area of red lesions (mm2) | 38, 46, 0, 0 (21 ± 24.5) | 17, 112, 0, 32 (40.3 ± 49.6) | 69, 1, 1, 83 (8.5 ± 43.7) | 9, 3, 7, 16 (8.7 ± 5.3) | 166,29,57,0 (62.9 ± 72.5) | 2, 0, 14, 0 (4 ± 6.7) |
| rAFS score | 8, 8, 8, 2 (6.5 ± 3) | 8, 8, 44, 2 (15.5 ± 19.2) | 8, 8, 8, 8 (8 ± 0) | 8, 8, 8, 8 (8 ± 0) | 4, 8, 8, 8 (7 ± 2) | 2, 8, 8, 8 (6.5 ± 3) |
| rAFS stage | 2, 2, 2, 1 (1.8 ± 0.5) | 2, 2, 4, 1 (2.3 ± 1.3) | 2, 2, 2, 2 (2 ± 2) | 2, 2, 2, 2 (2 ± 2) | 1, 2, 2, 2 (1.8 ± 0.5) | 1, 2, 2, 2 (1.8 ± 0.4) |
| No. adhesions | 3, 4, 4, 0 (2.8 ± 1.9) | 4, 2, 4, 0 (2.5 ± 1.9) | 2, 3, 3, 3 (2.8 ± 0.5) | 3, 4, 3, 3 (3.3 ± 0.5) | 0, 4, 7, 3 (3.5 ± 2.9) | 0, 3, 6, 4 (3.3 ± 2.5) |
Comparisons between the placebo, GnRH-antagonist and rosiglitazone groups after pre-and post-treatment laparoscopies. All data presented as raw individual values (mean ± standard deviation).
Table 2.
Specific lesion types and their changes for the various treatment groups.
| Placebo | GnRH-antagonist | Rosiglitazone | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Typical lesions | 1 (0-5) | 2.5 (0-7) | 1.5 (0-6) | 2.5 (1-4) | 0.5 (0-5) | 4 (1-11) |
| Red lesions | 1.5 (0-4) | 3 (0-6) | 2.5 (0-11) | 2 (1-5) | 5.5 (0-6) | 0.5 (0-2) |
| Typical and red lesions | 4 (0-9) | 5 (1-13) | 5.5 (2-13) | 6 (2-6) | 6 (0-11) | 4.5 (3-11) |
Number of typical and red endometriotic lesions in the placebo, GnRH-antagonist and rosiglitazone groups at the time of staging laparoscopy (pre-treatment) and final laparoscopy after 30 days of treatment (post-treatment). All data presented are median (range).
Fig 1. Gross appearance of endometriotic lesions at laparoscopy before and after treatment.

Laparoscopic appearance of endometriotic lesions in three representative baboons at staging laparoscopy on the left and 30 days after treatment shown on the right panels. The staging endometriotic lesions (arrows) range from white-orange plaques, red vesicles, typical blue-black to red polypoid excrescences. The final laparoscopy after treatment reveals black plaques, clear vesicles, and white fibrotic nodules (arrowheads). A) Baboon PAN 2985 before and after placebo treatment. B) Baboon PAN 2871 before and after Ganirelix treatment. C) Baboon PAN 3030 before and after rosiglitazone.
Surface areas of endometriotic lesions before and after treatment are shown in Figure 2 for each treatment group. The ratio of the surface area of each baboon at end of treatment compared to staging laparoscopy showed no significant difference between placebo and Ganirelix. The rosiglitazone-treated baboons did show a significant surface area difference from placebo-treated baboons (univariate ANOVA, post-hoc Dunnett t P=0.028, Tukey P=0.037, Bonferroni P=0.046). When assessing the relative change in surface area, the rosiglitazone treated group had a statistically significant reduction in surface area compared to placebo group (univariate ANOVA, post-hoc Dunnett t P=0.024, Tukey P=0.033, Bonferroni P=0.04; Figure 3). The Ganirelix group revealed statistical significant differences in relative change in surface area only with the Dunnett t post-hoc statistic (P=0.044).
Fig 2. Surface area boxplot.
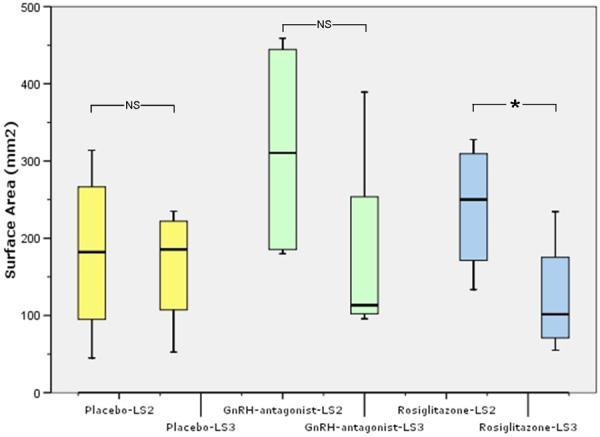
Box plot showing changes in total surface area (mm2) before and after the respective treatments, placebo, GnRH-antagonist, rosiglitazone (4 baboons/group). Horizontal small bars represent the 10-90th percentile range, and the boxes indicate the 25-75th percentile range. The horizontal line in each box corresponds to the median. (*, univariate ANOVA, post-hoc Dunnett t P=0.028, Tukey P=0.037, Bonferroni P=0.046; NS, non-significant)
Fig. 3. Relative change in surface area boxplot.
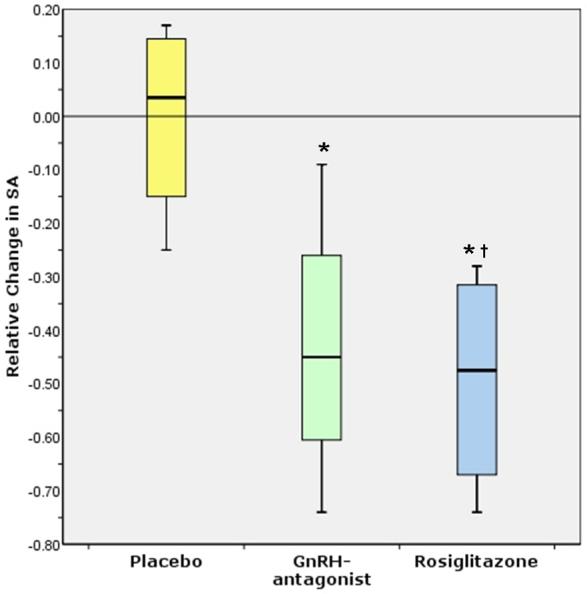
Box plot showing relative change in surface area (surface area after treatment minus surface area before divided by surface area before) for the three treatment groups (4 baboons/group): placebo, GnRH-antagonist, rosiglitazone. (*, univariate ANOVA, post-hoc Dunnett t P<0.05, †, post-hoc Dunnett t P=0.024, Tukey P=0.033, Bonferroni P=0.04)
Stack bar of mean number of lesions by lesion type (Figure 4) and mean surface area by lesion type (Figure 5) suggest a trend towards transformation of active lesions (red) to typical lesions in the rosiglitazone treatment in contrast to the placebo tablets or Ganirelix injected baboons. A paired t-test analysis of change in typical lesions before versus after 30-days of treatment in the placebo, GnRH-antagonist and rosiglitazone groups revealed 0.41, 0.84, 0.04, respectively. And paired t-tests for change in red lesions before versus after in each group, placebo, GnRH-antagonist and rosiglitazone, were 0.31, 0.38, 0.08, respectively. (Please see Table 2 for the median (range) of red and typical lesions in the three treatment groups.)
Fig. 4. Stack bar of mean number of lesions by lesion type.

Quantitative distribution of lesion types before (A) and after (B) the respective treatment for 30 days, placebo, GnRH-antagonist and rosiglitazone.
Fig. 5. Stack bar of mean surface area of lesions by lesion type.
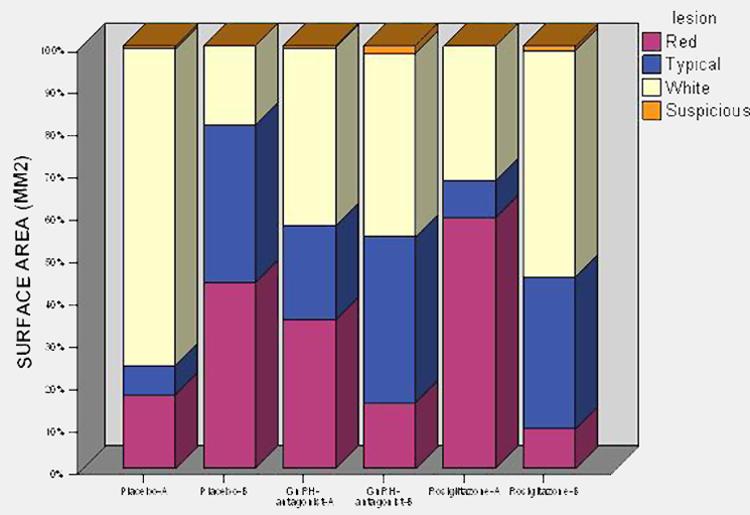
Distribution of the mean surface area by lesion types before (A) and after (B) the respective treatment for 30 days, placebo, GnRH-antagonist and rosiglitazone.
Effect of interventions and treatment on the perineal/menstrual cycle
The median number of cycling days between induction and the staging, day 30, laparoscopy was not significantly different in the three treatment groups (univariate ANOVA, data not shown). During the treatment month, three Ganirelix-treated baboons had no further menses and the remaining baboon sustained a withdrawal bleed.
Estradiol and progesterone serum levels
Median serum estradiol levels were equivalent in all groups at staging laparoscopy (median, range: 46.6, 23.4-60.8 pg/mL, univariate ANOVA, P>0.3) and post-treatment laparoscopy (median, range: 25.7, 15.1-43.8 pg/mL, univariate ANOVA, P>0.3, Figure 6). Within group analysis did not reveal any difference in estradiol level before or after treatment (paired t-tests, Figure 6).
Fig. 6. Serum hormone levels before and after drug treatment.
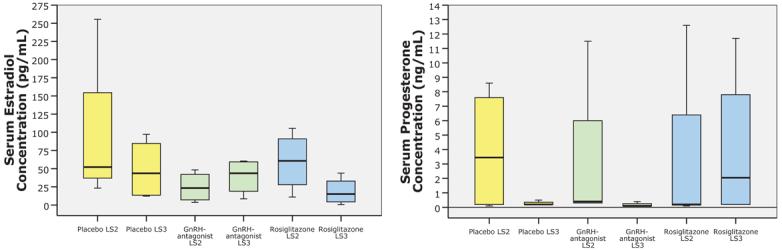
Box plot graphic of serum estradiol and progesterone concentrations in the three treatment groups (4 baboons/group) consisting of placebo, GnRH-antagonist and rosiglitazone at laparoscopy two (staging laparoscopy, LS2) and at laparoscopy three, 30 days after respective treatments (LS3). There was no significant difference in estradiol or progesterone concentration within groups before and after treatment or between groups at time of staging laparoscopy or at end of treatment laparoscopy (univariate ANOVA, P>0.3). Horizontal small bars represent the 10-90th percentile range, and the boxes indicate the 25-75th percentile range. The horizontal line in each box corresponds to the median.
Median serum progesterone levels were equivalent in all groups at staging laparoscopy (median, range: 0.3, 0.2-3.5 ng/mL) and post-treatment laparoscopy (median, range: 0.2, 0.1-2.1 ng/mL, univariate ANOVA, P>0.3, Figure 6). Within group analysis did not reveal any difference in progesterone levels before or after treatment (paired t-tests, Figure 6).
Discussion
Accumulating evidence supports the notion that the progression of endometriotic lesions is catalyzed by an aberrant immune response noted in the peritoneal cavity of women with endometriosis (23-30). It is uncertain if the initial development of the disease is related to this inflammatory response. However, retrograde menstruation or iatrogenic seeding of the peritoneum with endometrial tissue will incite an inflammatory reaction (31). This is manifested by greater amounts of activated macrophages, cytokines, chemokines and growth factors in the peritoneal fluid of endometriosis patients in contrast to controls (29, 32). The discovery that these lesions possess the functional aromatase enzyme whereas normal endometrium from control subjects does not (33), allows for a reconciliation of separate pathophysiologies—namely, the inflammatory component and the estrogen dependency of the disease. This union of processes is accomplished by the immune-induced synthesis of prostaglandin endoperoxidase synthase-2 leading to greater levels of prostaglandin E2, which, in turn, is a potent stimulator of the aromatase II promoter in endometriotic stromal cells (34).
PPAR-γ is a pleiotropic nuclear hormone receptor that binds to specific DNA response elements and may regulate gene expression indirectly, negatively or positively, through competition with other transcription factors (35). The true active endogenous PPAR-γ ligand has yet to be determined. The highest expression is found in adipose tissue where it is principally involved in adipocyte differentiation. Nevertheless, accumulating evidence purports a role for PPAR-γ ligands in regulating cell growth (36, 37), apoptosis (36, 38), inhibiting angiogenesis in human endometrial cells (39) and repressing inflammatory mediators (8, 13, 15, 40). In fact, most anti-inflammatory properties of PPAR-γ ligands are thought to arise through down-regulating proinflammatory mediators in macrophages (41) likely preceded by inhibiting the transcription factor nuclear factor-κB (NF-κB) (42-46). Since NF-κB appears to be constitutively activated in endometriotic cells and thus may play a central role in the pathophysiology of endometriosis (47), the utilization of an immunomodulatory drug such as rosiglitazone to ameliorate the immunological dysfunction seen with endometriosis represents an alternate novel treatment.
Since recent in vitro and animal model studies have implicated PPAR-γ ligands as potential modulators of inflammation-related diseases such as inflammatory bowel disease, psoriasis and rheumatoid arthritis, (48-50), their use in endometriosis is not far-fetched. Several in vitro studies with thiazolidinediones (TZDs) and endometriotic cells (15, 39) led to their use in endometriosis animal models. To date there have been two published studies using TZDs in the rat model of endometriosis with one revealing decreased induction of lesions (51) and another showing regression of established disease (19). Since rodents do not have menses or develop spontaneous endometriosis, the ectopic autologous transplantation of uterine tissue in this model (52) may not sufficiently replicate human endometriosis. With this in mind, the baboon endometriosis model was established by intrapelvic seeding of menstrual eutopic endometrium on top of the pelvic organs (20).
In the present study, a TZD, rosiglitazone, was used to assess if it could significantly diminish endometriotic lesions that were documented prior to treatment. The comparison groups were a placebo control, placebo treated baboons, and an active comparator or active control, GnRH-antagonist treated cohort. For two reasons a GnRH-antagonist was utilized rather than a GnRH-agonist: (A) due to a 30 day treatment, we wanted to avoid the agonist flare effect seen with a GnRH-agonist and (B) the IPR has experience with GnRH-antagonist administration for gonadal suppression used in baboon fertility studies. This study presents the first sub-human primate evidence that treatment with a TZD can reduce the surface area (∼50% decrease in relative change compared to placebo) of induced peritoneal endometriosis. Furthermore, our results advocate that rosiglitazone may augment the progression of endometriotic lesions from the more active red lesions to the older, typical, blue-black puckered implants though this did not reach statistical significance in our small sample size.
The staging laparoscopies were conducted at various times of the menstrual phase (Table 3) so the estradiol values were not illustrative of true suppression. However, by final laparoscopy, three of the Ganirelix baboons had progesterone levels below 0.21 ng/mL (below the sensitivity level of our assay) suggesting an ovarian suppressive effect while no other baboon in any other treatment group showed this low level. Moreover, two rosiglitazone baboons showed luteal phase levels of progesterone. The limited clinical studies on the reproductive influence of rosiglitazone are confined to the polycystic ovary syndrome population and reveal an improved ovulation rate and generous restoration of normal menstrual cycles (53-56). These references support our observation that there was no untoward endocrinological effect of rosiglitazone versus the placebo group.
Table 3.
Cycle day and perineal cycle stage with respect to surgeries.
| Animal # | Treatment | LS1 | CD | St, # | LS2 | CD | St, # | LS3 | CD | St, # | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PAN 2991 | P | 09/08/05 | 2 | 7, 2 | 11/15/05 | 24 | 0, 1 | 12/15/05 | 16 | 3, 3 |
| 2 | PAN 3014 | G | 09/10/05 | 1 | 7, 1 | 11/15/05 | 29 | 6, 2 | 12/15/05 | 21 | 3, 11 |
| 3 | PAN 3030 | R | 09/13/05 | 1 | 7, 1 | 11/15/05 | 16 | 4, 2 | 12/15/05 | 9 | 3, 3 |
| 4 | PAN 2985 | P | 09/15/05 | 1 | 7, 1 | 11/15/05 | 24 | 6, 2 | 12/15/05 | 16 | 3, 4 |
| 5 | PAN 2998 | R | 09/19/05 | 2 | 7, 2 | 11/16/05 | 19 | 2, 5 | 12/16/05 | 11 | 0, 2 |
| 6 | PAN 2631 | P | 09/19/05 | 2 | 7, 2 | 11/16/05 | 22 | 4, 5 | 12/16/05 | 12 | 4, 1 |
| 7 | PAN 3032 | R | 09/19/05 | 1 | 7, 1 | 11/16/05 | 19 | 3, 8 | 12/16/05 | 47 | 0, 4 |
| 8 | PAN 3036 | G | 10/03/05 | 1 | 7, 1 | 11/16/05 | 7 | 0, 4 | 12/16/05 | 37 | 3, 1 |
| 9 | PAN 2912 | P | 10/06/05 | 2 | 7, 2 | 11/17/05 | 8 | 3, 1 | 12/17/05 | 2 | 7, 2 |
| 10 | PAN 2871 | G | 10/06/05 | 1 | 7, 1 | 11/17/05 | 2 | 7, 2 | 12/17/05 | 32 | 3, 13 |
| 11 | PAN 2788 | R | 10/14/05 | 1 | 7, 1 | 11/17/05 | 35 | 0, 2 | 12/17/05 | 24 | 6, 1 |
| 12 | PAN 2993 | G | 10/14/05 | 1 | 7, 1 | 11/17/05 | 35 | 0, 10 | 12/17/05 | 28 | 0, 2 |
Menstrual phase cycle day and perineal cycle stage for all baboons at each surgical time frame.
P, placebo; G, GnRH-antagonist; R, rosiglitazone; LS, laparoscopy; CD, cycle day (counting from day 1 of menses); St, #, perineal cycle stage and number of days in that stage.
An ideal therapy option for treating endometriosis pain should possess a modicum of side effects, low-cost burden, proven efficacy at diminishing pelvic pain and spare fertility potential during treatment. Clinical data have exonerated rosiglitazone from the increased hepatoxicity risk as seen with other TZDs (57). Rosiglitazone is classified as a pregnancy category C drug due to animal evidence of growth retardation in mid to late gestation with 20 and 75 times the human dose. No evidence of teratogenicity exists in either preclinical or clinical trials. Rosiglitazone had no untoward effects on the growth and morphology of an in vitro rat embryo culture model despite concentrations as high as 10 times human peak plasma levels (58). Studies on early human pregnancy have shown that rosiglitazone can be transported across the placenta in the late phase of the first trimester from 8-to-12 weeks (59) but in an ex vivo human perfusion model there was negligible transfer of rosiglitazone across the placenta (60). This would suggest that it may be safe to take the TZD up until the time a pregnancy is confirmed.
Taken together, this study suggests that the PPAR-γ agonist rosiglitazone may reduce the quantitative burden of endometriotic disease in baboons with established disease without affecting the menstrual cycle. Moreover, the specific change from red lesions to more typical implants for rosiglitazone treated baboons compared to placebo or GnRH-antagonist groups hints at a possible conversion of active endometriotic lesions. Only further study in humans can determine if these results translate into diminished pelvic pain with rosiglitazone treatment.
Acknowledgement
The authors thank the staff of the Institute for Primate Research, for their generous contributions to the study. We also thank Elizabeth Ward, RN (University of Michigan, Ann Arbor, MI) for her assistance with the surgeries and documentation.
Acknowledgement of financial support
This research project was supported in part by NIH grant 5K23HD043952-02 (DIL) and by the University of Michigan Department of Obstetrics and Gynecology in part through the Ansbacher Fund for Resident/Fellow Education and Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. discussion 34-6, 396-406. [DOI] [PubMed] [Google Scholar]
- 2.Szendei G, Hernadi Z, Devenyi N, Csapo Z. Is there any correlation between stages of endometriosis and severity of chronic pelvic pain? Possibilities of treatment. Gynecol Endocrinol. 2005;21:93–100. doi: 10.1080/09513590500107660. [DOI] [PubMed] [Google Scholar]
- 3.D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–54. doi: 10.1055/s-2003-41330. [DOI] [PubMed] [Google Scholar]
- 4.Nothnick WB, Curry TE, Vernon MW. Immunomodulation of rat endometriotic implant growth and protein production. Am J Reprod Immunol. 1994;31:151–62. doi: 10.1111/j.1600-0897.1994.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 5.Ingelmo JM, Quereda F, Acien P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-alpha-2β in a murine model. Fertil Steril. 1999;71:907–11. doi: 10.1016/s0015-0282(99)00087-4. [DOI] [PubMed] [Google Scholar]
- 6.Keenan JA, Williams-Boyce PK, Massey PJ, Chen TT, Caudle MR, Bukovsky A. Regression of endometrial explants in a rat model of endometriosis treated with the immune modulators loxoribine and levamisole. Fertil Steril. 1999;72:135–41. doi: 10.1016/s0015-0282(99)00157-0. [DOI] [PubMed] [Google Scholar]
- 7.D’Antonio M, Martelli F, Peano S, Papoian R, Borrelli F. Ability of recombinant human TNF binding protein-1 (r-hTBP-1) to inhibit the development of experimentally-induced endometriosis in rats. J Reprod Immunol. 2000;48:81–98. doi: 10.1016/s0165-0378(00)00073-5. [DOI] [PubMed] [Google Scholar]
- 8.Hornung D, Chao VA, Vigne JL, Wallwiener D, Taylor RN. Thiazolidinedione inhibition of peritoneal inflammation. Gynecol Obstet Invest. 2003;55:20–4. doi: 10.1159/000068952. [DOI] [PubMed] [Google Scholar]
- 9.Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst AM. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril. 2004;81(Suppl 1):775–9. doi: 10.1016/j.fertnstert.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 10.D’Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, et al. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74:131–6. doi: 10.1095/biolreprod.105.043349. [DOI] [PubMed] [Google Scholar]
- 11.Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21:1856–62. doi: 10.1093/humrep/del044. [DOI] [PubMed] [Google Scholar]
- 12.Hornung D, Waite LL, Ricke EA, Bentzien F, Wallwiener D, Taylor RN. Nuclear peroxisome proliferator-activated receptors alpha and gamma have opposing effects on monocyte chemotaxis in endometriosis. J Clin Endocrinol Metab. 2001;86:3108–14. doi: 10.1210/jcem.86.7.7615. [DOI] [PubMed] [Google Scholar]
- 13.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 14.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100:6712–7. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-gamma decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. 2002;87:1841–4. doi: 10.1210/jcem.87.4.8409. [DOI] [PubMed] [Google Scholar]
- 16.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996;12:335–63. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 18.Fan W, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, et al. Activation of peroxisome proliferator-activated receptor-gamma and retinoid X receptor inhibits aromatase transcription via nuclear factor-kappaB. Endocrinology. 2005;146:85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 19.Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82(Suppl 3):1008–13. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- 20.D’Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) Am J Obstet Gynecol. 1995;173:125–34. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 21.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 22.Kriewaldt FH, Hendrickx AG. Reproductive parameters of the baboon. Lab Anim Care. 1968;18:361–70. [PubMed] [Google Scholar]
- 23.Akoum A, Kong J, Metz C, Beaumont MC. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. 2002;77:989–94. doi: 10.1016/s0015-0282(02)03082-0. [DOI] [PubMed] [Google Scholar]
- 24.Keenan J, Chen T, Chadwell N. IL-1β, TNF-α, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 1995;34:381–5. doi: 10.1111/j.1600-0897.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 25.Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85:824–9. doi: 10.1210/jcem.85.2.6335. [DOI] [PubMed] [Google Scholar]
- 26.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–8. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 27.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–9. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Expression of interleukin-1 (IL-1) beta messenger ribonucleic acid (mRNA) and IL-1 receptor antagonist mRNA in peritoneal macrophages from patients with endometriosis. Fertil Steril. 1992;57:535–42. doi: 10.1016/s0015-0282(16)54896-1. [DOI] [PubMed] [Google Scholar]
- 29.Lebovic DI, Mueller MD, Hornung D, Taylor RN. Immunology of endometriosis. Immunol Allergy Clin N Am. 2002;22:585–98. [Google Scholar]
- 30.Mueller MD, Mazzucchelli L, Buri C, Lebovic DI, Dreher E, Taylor RN. Epithelial neutrophil-activating peptide 78 concentrations are elevated in the peritoneal fluid of women with endometriosis. Fertil Steril. 2003;79(Suppl 1):815–20. doi: 10.1016/s0015-0282(02)04828-8. [DOI] [PubMed] [Google Scholar]
- 31.D’Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus) Am J Obstet Gynecol. 2001;184:917–25. doi: 10.1067/mob.2001.111715. [DOI] [PubMed] [Google Scholar]
- 32.Cirkel U, Ochs H, Mues B, Zwadlo G, Sorg C, Schneider HP. Inflammatory reaction in endometriotic tissue: an immunohistochemical study. Eur J Obstet Gynecol Reprod Biol. 1993;48:43–50. doi: 10.1016/0028-2243(93)90052-e. [DOI] [PubMed] [Google Scholar]
- 33.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–9. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 34.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 35.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–9. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yee LD, Sabourin CL, Liu L, Li HM, Smith PJ, Seewaldt V, et al. Peroxisome proliferator-activated receptor gamma activation in human breast cancer. Int J Oncol. 1999;15:967–73. doi: 10.3892/ijo.15.5.967. [DOI] [PubMed] [Google Scholar]
- 37.Houston KD, Copland JA, Broaddus RR, Gottardis MM, Fischer SM, Walker CL. Inhibition of proliferation and estrogen receptor signaling by peroxisome proliferator-activated receptor gamma ligands in uterine leiomyoma. Cancer Res. 2003;63:1221–7. [PubMed] [Google Scholar]
- 38.Heaney AP, Fernando M, Melmed S. PPAR-gamma receptor ligands: novel therapy for pituitary adenomas. J Clin Invest. 2003;111:1381–8. doi: 10.1172/JCI16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPARgamma represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2005;8:373–9. doi: 10.1007/s10456-005-9027-4. [DOI] [PubMed] [Google Scholar]
- 40.Wanichkul T, Han S, Huang RP, Sidell N. Cytokine regulation by peroxisome proliferator-activated receptor gamma in human endometrial cells. Fertil Steril. 2003;79(Suppl 1):763–9. doi: 10.1016/s0015-0282(02)04835-5. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005;54:464–70. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- 42.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–80. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Anderson PO, Chen S, Paulsson KM, Sjogren HO, Li S. Inhibition of the transcription factors AP-1 and NF-kappaB in CD4 T cells by peroxisome proliferator-activated receptor gamma ligands. Int Immunopharmacol. 2001;1:803–12. doi: 10.1016/s1567-5769(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 44.Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631–46. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 45.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–59. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 46.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 47.Guo SW. Nuclear Factor-kappaB (NF-kappaB): An Unsuspected Major Culprit in the Pathogenesis of Endometriosis That Is Still at Large? Gynecol Obstet Invest. 2006;63:71–97. doi: 10.1159/000096047. [DOI] [PubMed] [Google Scholar]
- 48.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Curr Med Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 49.Lewis JD, Lichtenstein GR, Stein RB, Deren JJ, Judge TA, Fogt F, et al. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001;96:3323–8. doi: 10.1111/j.1572-0241.2001.05333.x. [DOI] [PubMed] [Google Scholar]
- 50.Bongartz T, Coras B, Vogt T, Scholmerich J, Muller-Ladner U. Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford) 2005;44:126–9. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 51.Demirturk F, Aytan H, Caliskan AC, Aytan P, Koseoglu DR. Effect of peroxisome proliferator-activated receptor-gamma agonist rosiglitazone on the induction of endometriosis in an experimental rat model. J Soc Gynecol Investig. 2006;13:58–62. doi: 10.1016/j.jsgi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–94. [PubMed] [Google Scholar]
- 53.Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 2003;79:562–6. doi: 10.1016/s0015-0282(02)04843-4. [DOI] [PubMed] [Google Scholar]
- 54.Cataldo NA, Abbasi F, McLaughlin TL, Basina M, Fechner PY, Giudice LC, et al. Metabolic and ovarian effects of rosiglitazone treatment for 12 weeks in insulin-resistant women with polycystic ovary syndrome. Hum Reprod. 2006;21:109–20. doi: 10.1093/humrep/dei289. [DOI] [PubMed] [Google Scholar]
- 55.Shobokshi A, Shaarawy M. Correction of insulin resistance and hyperandrogenism in polycystic ovary syndrome by combined rosiglitazone and clomiphene citrate therapy. J Soc Gynecol Investig. 2003;10:99–104. doi: 10.1016/s1071-5576(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 56.Sepilian V, Nagamani M. Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance. J Clin Endocrinol Metab. 2005;90:60–5. doi: 10.1210/jc.2004-1376. [DOI] [PubMed] [Google Scholar]
- 57.Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care. 2002;25:815–21. doi: 10.2337/diacare.25.5.815. [DOI] [PubMed] [Google Scholar]
- 58.Chan LY, Lau TK. Effect of rosiglitazone on embryonic growth and morphology: a study using a whole rat embryo culture model. Fertil Steril. 2006;86:490–2. doi: 10.1016/j.fertnstert.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 59.Chan LY, Yeung JH, Lau TK. Placental transfer of rosiglitazone in the first trimester of human pregnancy. Fertil Steril. 2005;83:955–8. doi: 10.1016/j.fertnstert.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 60.Holmes HJ, Casey BM, Bawdon RE. Placental transfer of rosiglitazone in the ex vivo human perfusion model. Am J Obstet Gynecol. 2006;195:1715–9. doi: 10.1016/j.ajog.2006.03.054. [DOI] [PubMed] [Google Scholar]


