Abstract
Objective:
A previous study from this laboratory showed that elevation of endogenous angiotensin II (Ang II) and upregulation of the angiotensin II type 1 (AT1) receptor in the carotid body (CB) are involved in the enhanced peripheral chemoreceptor sensitivity in rabbits with chronic heart failure (CHF). NADPH oxidase-derived superoxide anion mediates the effects of Ang II in many organs. We investigated whether this signaling pathway may mediate the enhanced peripheral chemoreceptor sensitivity induced by Ang II in CHF rabbits.
Methods and results:
By recording single-unit activity from the carotid sinus nerve in isolated preparations, we found that phenylarsine oxide 2 μM (PAO, NADPH oxidase inhibitor) and TEMPOL 1 mM (superoxide dismutase mimetic) significantly decreased not only the Ang II-enhanced CB chemoreceptor responses to different levels of hypoxia in sham rabbits (Δ-12.5 ± 0.8 and Δ-12.8 ± 0.9 imp/s at 40.7 ± 2.3 mm Hg of PO2, and Δ-5.6 ± 0.5 and Δ-5.3 ± 0.4 imp/s at 60.2 ± 3.1 mm Hg of PO2, p<0.05, respectively) but also the CHF-induced elevation of CB chemoreceptor responses to different levels of hypoxia (Δ-13.6 ± 1.1 and Δ-13.7 ± 0.9 imp/s at 40.9 ± 3.1 mm Hg of PO2, and Δ-6.7 ± 1.2 and Δ-6.6 ± 0.8 imp/s at 59.8 ± 3.5 mm Hg of PO2, p<0.05). In addition, mRNA and protein expressions of NADPH oxidase components (gp91phox, p40phox and p47phox) were higher in the CB from CHF rabbits compared to sham rabbits. Furthermore, 100 pM Ang II induced an increase in superoxide production in CB homogenates from sham rabbits, which was similar to that in CB homogenate from CHF rabbits. PAO and Tempol inhibited the Ang II- and CHF-enhanced superoxide anion production.
Conclusions:
These results suggest that the enhanced peripheral chemoreceptor sensitivity mediated by Ang II in CHF rabbits occurs via a NADPH oxidase-superoxide signaling pathway.
Keywords: Angiotensin, Reactive oxygen species, autonomic nervous system, chemoreceptor, heart failure
1. INTRODUCTION
In chronic heart failure (CHF), sympathetic nerve function is enhanced, which contributes to long-term deterioration of cardiac function [1-3]. In addition to attenuated baroreflex sensitivity [1, 3], the enhanced sensitivity of the peripheral chemoreflex mediates sympathetic hyperactivity in the CHF state [4-5]. An augmented afferent input from carotid body (CB) chemoreceptors is involved in the enhancement of peripheral chemoreflex function in rabbits with CHF [6].
It is well documented that systemic and tissue Ang II levels are increased in CHF patients and in animal models of CHF [8-11]. Ang II increases CB chemoreceptor activity via the AT1 receptor [12]. Furthermore, we have shown that elevation of Ang II and concomitant upregulation of Ang II type 1 (AT1) receptors in the CB contribute to the enhanced CB chemoreceptor sensitivity in the CHF state [13].
However, the mechanism by which Ang II enhances the CB chemoreceptor sensitivity to hypoxia and peripheral chemoreflex function in CHF is not known. Ang II activates NADPH oxidase via the AT1 receptor [14], which serves as a significant source of intracellular reactive oxygen species (ROS) in many tissues [15-20]. Furthermore, a significant body of evidence indicates that the components of the NADPH oxidase are present in peripheral chemoreceptor sites including pulmonary neuroepithelial bodies and CBs [21-23]. We hypothesized that NADPH oxidase-derived superoxide anion mediates the Ang II-enhanced CB chemoreceptor sensitivity in rabbits with CHF. In this study, therefore, we measured: (1) mRNA and protein expression of NADPH oxidase components in the CB from sham and CHF rabbits; (2) NADPH-derived superoxide anion production in the CB from sham and CHF rabbits; (3) the effects of NADPH oxidase inhibitors and superoxide scavengers on the Ang II- or CHF-enhanced CB chemoreceptor sensitivity [13].
2. METHODS
2.1. Pacemaker implant and production of CHF
All experiments were carried out on male New Zealand White rabbits weighing 2.5-4.0 Kg. Experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the American Physiological Society's Guides for the Care and Use of Laboratory Animals. Rabbits were assigned to sham-operated and CHF groups. They were housed in individual cages under controlled temperature and humidity and a 12:12-h dark-light cycle, and fed standard rabbit chow with water available ad libitum.
Rabbits were anesthetized with a cocktail consisting of 1.2 mg acepromazine, 5.9 mg xylazine and 58.8 mg ketamine, given as an i.m. injection. Using sterile technique, a left thoracotomy was performed as previously described [4]. Briefly, a pin electrode was attached to the left ventricle for pacing. Two sonomicrometer crystals (Sonometrics Corp., London, ON, Canada) were attached to the anterior and posterior walls of the lateral left ventricle for measuring external diameter. The chest was closed. Rabbits were placed on an antibiotic regimen consisting of 5 mg/kg Baytril i.m. for 5 days. After 2 weeks, baseline left-ventricular end-systolic and end-diastolic external diameter (D), fractional shortening, and shortening velocity (dD/dt max) were measured by sonomicrometry (Triton Technology Inc., San Diego, CA, USA). Sonograms and experimental procedures were performed with the pacemaker turned off for at least 60 min before the recordings were started. The pacing was started at 320 bpm, held for 7 days, and then the rate was gradually increased to 380 bpm, with an increment of 20 bpm each week. Rabbits were paced for 3-4 weeks, which resulted in >40% reductions in dD/dtmax and shortening fraction (Table 1). This level of left ventricular dysfunction in paced rabbits has been consistently observed in our previous studies [4,6,13,24,25]. It is comparable to moderate compensated CHF in humans (Class III) and is accompanied by chronic sympathetic activation [4] and enhanced CB chemoreflex function [6]. Sham-operated animals underwent a similar period of sonographic measurements with the pacemaker turned off. Any rabbit exhibiting abnormal arterial blood gases (PaO2 < 85 mm Hg; 45 mm Hg < PaCO2 < 30 mm Hg) were excluded from study [4].
Table 1.
Cardiac diameters and contractility in sham and CHF rabbits
| Sham (n=25) |
CHF (n=25) |
|||
|---|---|---|---|---|
| Baseline | 3-4 wk | Baseline | 3-4 wk | |
| ESD, % of control | 100 ± 0.4 | 100.3 ± 1.1 | 100 ± 0.3 | 115.7 ± 1.9*# |
| EDD, % of control | 100 ± 0.3 | 100.1 ± 0.9 | 100 ± 0.3 | 109.7 ± 1.5*# |
| dD/dtmax, mm/s | −10.2 ± 1.0 | −10.4 ± 0.8 | −10.3 ± 0.7 | −4.0 ± 0.8*# |
| % shortening | 10.3 ± 0.8 | 10.3 ± 0.9 | 10.1 ± 1.0 | 3.9 ± 0.7*# |
Data are means ± SEM; ESD, left ventricular end-systolic diameter; EDD, left ventricular end-diastolic diameter; dD/dtmax, 1st derivative of change in diameter; %shortening=(EDD-ESD)/EDD×100%.
P<0.05 vs. baseline
p<0.05 vs. sham rabbits at the same period.
2.2. CB Chemoreceptor Fiber Activity
Single unit action potentials were recorded from CB chemoreceptor fibers in the carotid sinus nerve (CSN) as we have described previously [6]. Briefly, the left or right carotid sinus region was vascularly isolated and perfused with Krebs-Henseleit solution (in mM: 120 NaCl, 4.8 KCl, 2.0 CaCl, 2.5 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 5.5 glucose; 10 ml/min, T 37°C). Perfusate was bubbled with O2/CO2/N2 gas mixture at proper proportions (∼15%/5%/80%) to maintain PO2 at 100-110 mm Hg, PCO2 at 30-35 mm Hg, and pH at 7.4 as the normoxic condition. PO2, PCO2 and pH of the buffer solution perfusing the carotid sinuses were measured by gas- and ion-selective electrodes with 1201 chemical microsensor (Diamond General, Ann Arbor, MI, USA). Flow through the isolated sinus was set at 10 ml/min at a perfusion pressure of 80 mm Hg via a screw clamp on the effluent line, which was adjusted throughout the experiments to keep flow and pressure constant.
Autonomic innervation to the carotid sinus region was eliminated by stripping all visible neural connections among the carotid sinus, the superior cervical, and nodose ganglia. The CSN was exposed and transected near the petrosal ganglion to interrupt neural efferents to the CB.
The CSN was covered with mineral oil, and fine slips of nerve filaments were placed on a silver electrode. Impulses were amplified with a bandwidth of 100 Hz-3 kHz (Grass P511, Grass Instrument, Quincy, MA, USA), displayed on an oscilloscope (2120 Oscilloscope, BK Precision, Taiwan), and fed into a rate meter (FHC, Brunswick, ME, USA) whose window discriminators were set to accept potentials of the particular amplitude. Impulses were counted by the ratemeter in 1-sec bins. The action potential and ratemeter signals (discharge frequency) were fed into an analog-to-digital converter (ADInstruments, Colorado Springs, CO, USA) attached to a computer. Bundles that had one, or at most two, easily distinguishable active fibers were used. Chemoreceptor afferents were identified by their sparse and irregular discharge at normoxia and by their response to hypoxia and NaCN.
PO2 was altered by bubbling the perfusate with gas mixtures of different O2 concentrations (approximate 15, 8, and 4%) achieve a PO2 of 100-110, 55-65, and 35-40 mm Hg, respectively. The gas mixtures contained a constant fraction of ∼5% CO2 (30-35 mm Hg) and a balance of N2.
2.3. Semi-quantitative RT-PCR for NADPH oxidase components
CBs were rapidly removed and immediately frozen in dry ice and stored at −80°C until analyzed. The detailed procedures of RT-PCR for NADPH oxidase component mRNA have been reported [20]. Briefly total RNA was isolated by means of the RNeasy Mini Kit Total RNA Isolation System (QIAGEN Inc., Valencia, CA, USA), and then cDNA was synthesized by means of M-MLV Reverse Transcriptase (Invitrogen Life technologies, Carisbad, CA, USA), according to the manufacturer's instructions. PCR amplification was performed by means of a PTC-100 Programmable Thermal Controller (MJ Research, Inc., Watertown, MA, USA) as follows: 1 cycle at 95°C for 15 min, followed by 35 cycles of 94°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min. The primer pairs were based on the cDNA sequences of rabbit gp91phox (GenBank-AF323788), p67phox (GenBank-AF323789), p47phox (GenBank-AF324409), p40phox (GenBank-AF323790), and p22phox (GenBank-AF323787) with β–actin (GenBank-AF309819) as an internal control. The primer pairs were 5'-GCTTGTGGCTGTGATAAGCA-3' and 5'-CTCCTGCATCTGTGTCTCCA-3' for gp91phox, 5'-AACTCAGTGGGTGACCAAGG-3' and 5'-GTTCTTCCACGAAGGCTCTG-3' for p67phox, 5'-ACGAGAGTGGTTGGTGGTTC-3' and 5'-TAGCCAGTGACGTCCTCCTT-3' for p47phox, 5'-TCACTGGGAACAGCAAACTG-3' and 5'-CTGCTGAGGTCTTCCTCCAC-3' for p40phox, 5'-CGCTTCACCCAGTGGTACTT-3' and 5'-GCAGCCAGCAGGTAGATGAT-3' for p22phox, and 5'-GATCGC-TGACCGTATGCAG-3' and 5'-GTCGTACTCCTGCTTGGTG-3' for β–actin. The amplification products were visualized on 2% agarose gels by the use of ethidium bromide and sequenced so that their identity could be confirmed. The bands were analyzed using UVP BioImaging Systems (UVP Inc, Upland, CA, USA).
2.4. Western blot analysis for NADPH oxidase components
CBs were obtained in the same manner as described above. The protein was extracted with the lysing buffer (10 mM PBS, 1 % Nonidet P-40, 0.5 % sodium deoxycholate, 1 % SDS) plus protease inhibitor cocktail (100 μl/ml). Following a centrifugation at 12000 g for 20 min at 4 °C, the protein concentration in the supernatant was determined using a BCA protein assay kit (Pierce Chemical, Rockford, IL, USA). The protein sample was mixed with loading buffer containing β-mercaptoethanol and heated at 100°C for 5 min. Five μg of protein was loaded. Protein was fractionated in a 10% polyacrylamide gel along with molecular weight standards and transferred to PVDF membrane. The membrane was probed with goat anti gp91phox, p67phox, p47phox, p40phox and p22phox antibodies (Santa Cruz, CA, USA) and a peroxidase-conjugated rabbit anti-goat IgG (Santa Cruz, CA, USA). The signal was detected using enhanced chemiluminescence substrate (Pierce Chemical, Rockford, IL, USA) and the bands were analyzed using UVP BioImaging Systems. Protein loading was controlled by probing all Western blots with mouse anti-GAPDH antibody (Santa Cruz, CA, USA) and normalizing gp91phox, p67phox, p47phox, p40phox and p22phox protein intensities to that of GAPDH.
2.5. In situ detection of superoxide anion production and tyrosine hydroxylase (TH)
Fresh CB was frozen and cut into 15 μM-thick sections and arranged in separate wells of a 24-well plate in PBS solution with 5 μM dihydroethidium (DHE, Molecular Probes, Carisbad, CA, USA). Plates were incubated in the dark at 37°C for 30 min. After placed on glass slides, the sections were post-fixed in mixing solution of ethanol and methanol (volume 1:1) for 20 min at 4°C and then incubated with 10% of normal donkey serum for 1 hour followed by incubation with primary goat anti-TH antibody (Santa Cruz, CA, USA) for 4 hours at room temperature. Then the sections were incubated with donkey anti-goat secondary antibody (Molecular Probe, Carisbad, CA, USA) for 60 min at room temperature. At last, slides were observed under a Leica fluorescent microscope with appropriate excitation/emission filters. Pictures were captured by a digital camera system. No staining was seen when the procedure described above was used but PBS was used instead of the primary antibody.
2.6. Measurement of superoxide anion production
CB samples were homogenized and centrifuged (3000 rpm) at 4°C for 5 min. The supernatant was used to measure superoxide anion production. Total protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce; Rockford, IL). Superoxide ion production was measured using lucigenin chemiluminescence method [26-28]. The supernatant was placed in 0.5 ml microfuge containing dark–adapted lucigenin (5 μM), then read in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) with and without NADPH (100 μM). Light emission was recorded for 5 min and expressed as mean light units (MLU)/min/100 μg protein. In order to investigate the effects of some drugs on the superoxide anion production, the homogenates were pretreated for 30 min with one or combination of the following drugs: (1) Ang II (100 pM); (2) AT1 receptor antagonist, L158,809 (1 μM); (3) the NADPH oxidase inhibitors, phenylarsine oxide (PAO, 2 μM) or apocynin (APO, 100 μM); (4) SOD mimetic, tempol (1 mM).
2.7. Drugs
L-158,809 was a gift from Merck Co., NJ, USA. Tempol was purchased from Alexis Biochemicals Co., CA, USA. APO was purchased from CALBIOCHEM, CA, USA. Other chemicals used in this study were obtained from Sigma-Aldrich Chemical Co., St. Louis, MO, USA.
2.8. Data analysis
All data are presented as means ± SEM. Statistical significance was determined by student's paired t test for hemodynamic parameters, and student's unpaired t test for mRNA and protein expression of NADPH oxidase components. A two-way ANOVA, with a Bonferroni procedure for post hoc was used in comparisons of single-unit activity of CB chemoreceptor afferent nerve and superoxide production. Statistical significance was accepted when p<0.05. A power analysis was conducted to assess whether the sample size was sufficient to insure p<0.05.
3. Results
3.1.Induction of CHF
Rapid left ventricular pacing induced CHF by the 3rd or 4th week. LV dD/dtmax and LV shortening fraction were reduced by 59.7 ± 2.2% and 60.5 ± 2.4%, respectively, after 3 or 4 weeks of pacing compared to prepaced baselines (p<0.05, Table 1). There was no significant change in the LV dD/dtmax and LV shortening fraction between the baseline and the 3rd or 4th week in sham rabbits (Table 1).
3.2. Effects of L-158,809, PAO, and tempol on Ang II-enhanced CB chemoreceptor activity in sham rabbits
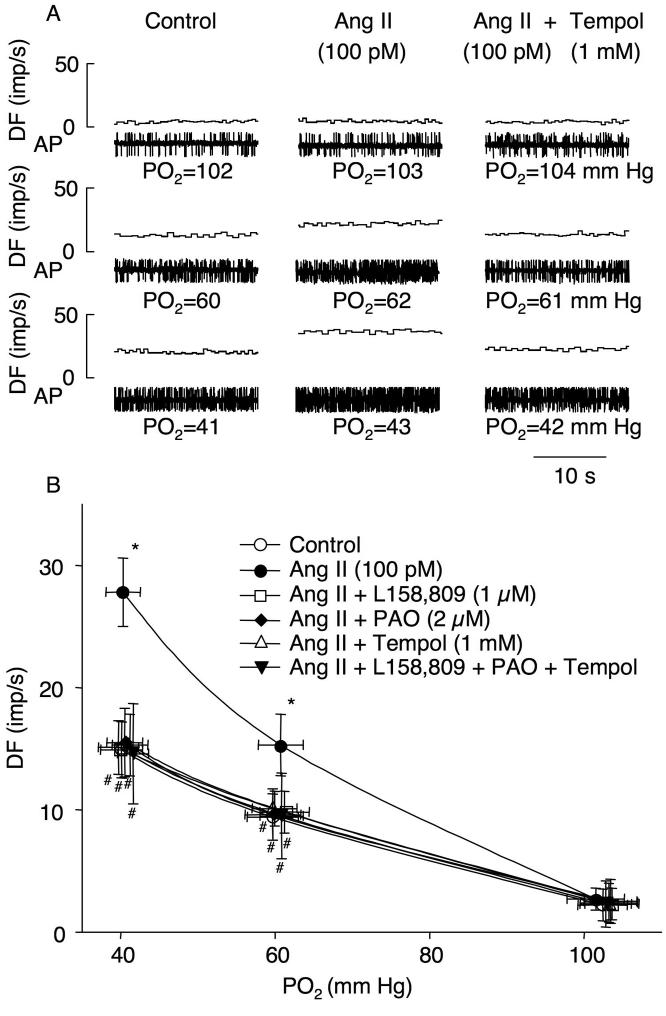
In the isolated perfused preparation of the carotid sinus region, administration of Ang II (100 pM) did not change the baseline CB chemoreceptor activity (3.0 ± 1.3 control vs. 3.5 ± 0.9 imp/s Ang II), but it augmented the CB chemoreceptor response to different levels of hypoxia (Fig. 1), which is consistent with data from our previous study [13]. Pretreatment of L-158,809 (1 μM), PAO (2 μM), or tempol (1 mM), significantly blunted the effect of Ang II to increase the CB chemoreceptor response to hypoxia and the combined benefits of L-158,809, PAO, and tempol on the effect of Ang II were non-additive (Fig. 1). These three inhibitors alone (without Ang II) did not show any effect on the CB chemoreceptor activity at normoxia or during hypoxia in sham rabbits. APO (100 μM), another NADPH oxidase inhibitor, also attenuated the Ang II-enhanced CB chemoreceptor sensitivity to hypoxia with effects similar to PAO (n= 5, data not shown).
Figure 1.
Involvement of NADPH oxidase-derived superoxide in Ang II-enhanced CB chemoreceptor afferent discharge at different oxygen levels in sham rabbits. A. Representative recordings of CB chemoreceptor afferent discharge in control, perfusion with 100 pM Ang II alone, and with 100 pM Ang II plus 1 mM tempol in sham rabbits. B. Effects of L158,809 (AT1 receptor antagonist), phenylarsine oxide (PAO, NADPH oxidase inhibitor), and tempol (superoxide dismutase mimic) on Ang II-enhanced CB chemoreceptor sensitivity in sham rabbits. Data are means ± SEM, n=7 in each group. *p<0.05 vs control; #p<0.05 vs Ang II. DF, discharge frequency; AP, action potential; PO2, oxygen partial pressure.
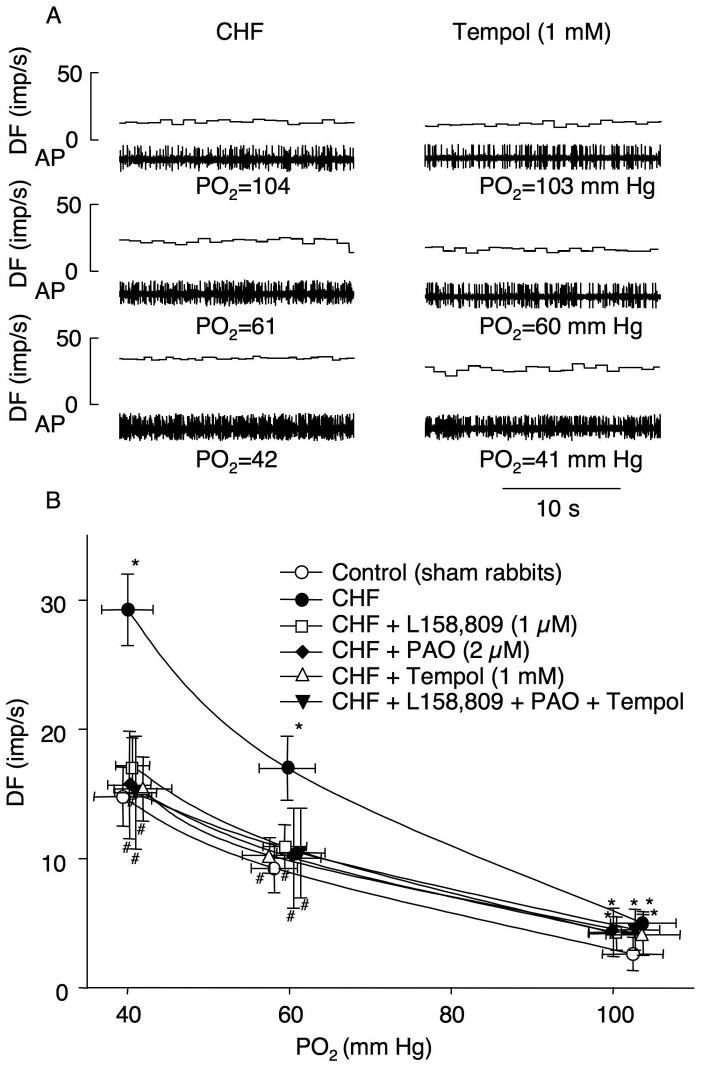
3.3. Effects of L-158,809, PAO, and tempol on CB chemoreceptor activity in CHF rabbits
The baseline CB chemoreceptor activity at normoxia (6.2 ± 0.9 imp/s) and its responses to the different levels of isocapnic hypoxia were enhanced in CHF rabbits (P < 0.05), compared with that in sham rabbits (Fig. 2), and as reported in our previous studies [6, 13]. L158,809, PAO, or tempol alone did not affect CHF-enhanced CB chemoreceptor activity at normoxia, whereas they markedly decreased the CB chemoreceptor responses to the graded levels of hypoxia in CHF rabbits towards the level observed in sham rabbits (Fig. 2). Similarly, APO also inhibited the CB chemoreceptor excitation to hypoxia in CHF rabbits (n= 5, data not shown).
Figure 2.
Intermediary effect of NADPH oxidase-derived superoxide in CHF-enhanced CB chemoreceptor afferent discharge at different oxygen levels in CHF rabbits. A. Representative recordings of CB chemoreceptor afferent discharge in control and perfusion 1 mM tempol. B. Effects of L158,809, PAO, and tempol on the CB chemoreceptor sensitivity in CHF rabbits. Data are means ± SEM, n=7 in each group. *P<0.05 vs sham rabbits; #p<0.05 vs CHF. DF, discharge frequency; AP, action potential; PO2, oxygen partial pressure.
3.4. mRNA and protein expression of NADPH oxidase components in CBs from sham and CHF rabbits
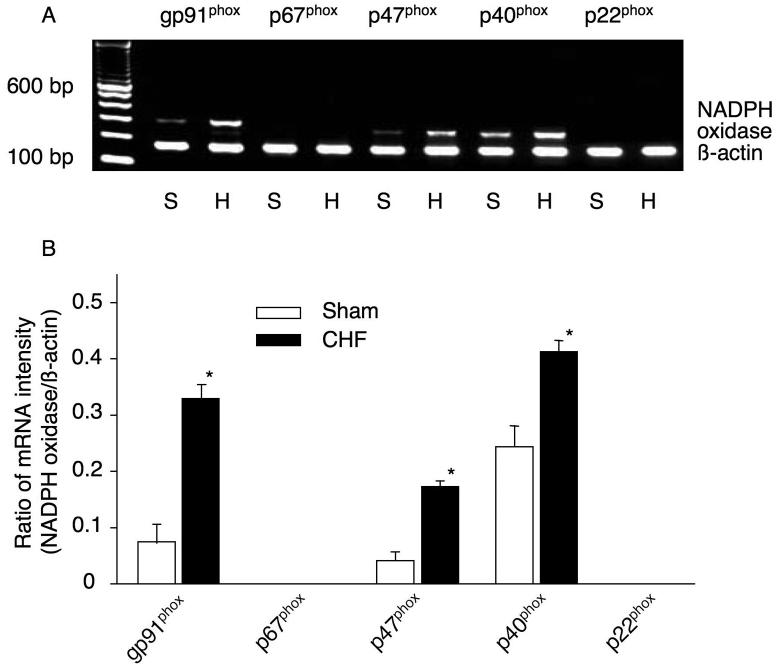
NADPH oxidase is a complex enzyme consisting of two membrane-bound components (gp91phox and p22phox) and three cytosolic components (p67phox, p47phox, and p40phox) [29], which was first found in phagocytic cells [30]. RT-PCR analysis of NADPH oxidase components in CBs of rabbits is shown in Fig. 3. The mRNA of gp91phox, p47phox and p40phox were expressed in the CBs. However, mRNA expression of p67phox and p22phox could not be detected in the CBs although we could detect the mRNA expression of p67phox and p22phox in liver and kidney from sham rabbits as a positive control (data not shown). Furthermore, the mRNA expression of gp91phox, p47phox and p40phox in the CBs from CHF rabbits was higher than that from sham rabbits (Fig. 3).
Figure 3.
mRNA expression of NADPH oxidase components in the CBs from sham and CHF rabbits. The representative (A) and summary (B) results for mRNA expression of NADPH oxidase components. Data are means ± SEM, n=7 in each group. *P<0.05 vs sham rabbits.
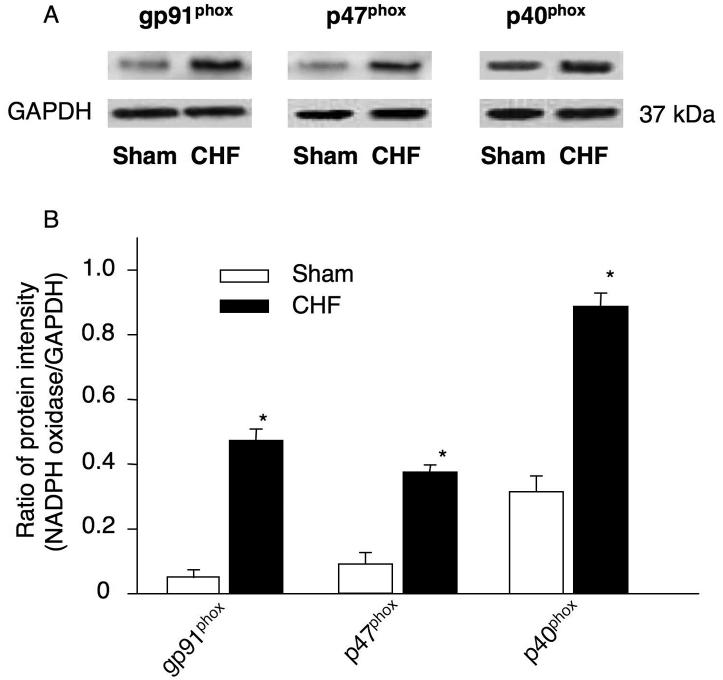
In addition, the protein expression of gp91phox, p47phox and p40phox in the CBs was also elevated in the CB from CHF rabbits, compared with that in sham rabbits (Fig. 4). But neither p67phox nor p22phox protein was detected in the CBs from either sham or CHF rabbits.
Figure 4.
Protein expression of NADPH oxidase components in the CBs from sham and CHF rabbits. The representative (A) and summary (B) results for protein expression of NADPH oxidase components. Data are means ± SEM, n=7 in each group. *P<0.05 vs sham rabbits.
3.5. Superoxide anion production in CBs from sham and CHF rabbits
Using a double-staining technique, we observed that a low level production of superoxide anion was present in the glomus cell clusters of the CB from sham rabbits (Fig. 5). Glomus cells were identified by the immunostaining of TH as a positive marker for the CB glomus cells [31]. After 30 min of Ang II pretreatment (100 pM), superoxide anion production increased in the CB glomus cells from sham rabbits (Fig. 5). Superoxide anion production was higher in the CB glomus cells from CHF rabbits than that from sham rabbits. L158,809 (1 μM) decreased the superoxide anion production in the CB glomus cells from CHF rabbits (Fig. 5).
Figure 5.
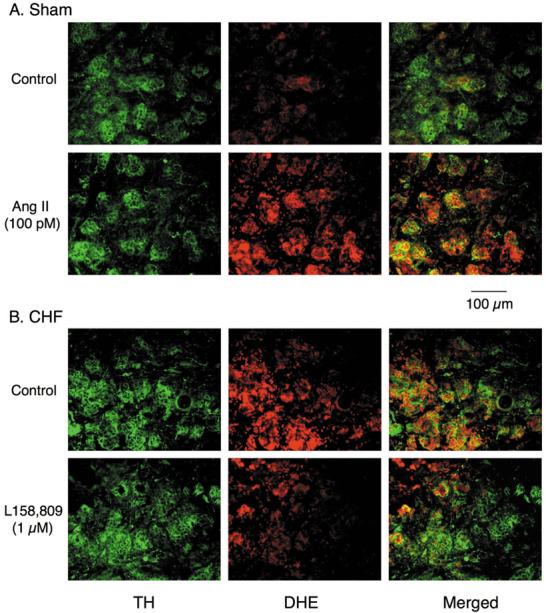
Superoxide anion production in CB glomus cells from sham and CHF rabbits, as assessed by dihydroethidium (DHE) fluorescence. Tyrosine hydroxylase (TH) serves as a positive marker for the CB glomus cells.
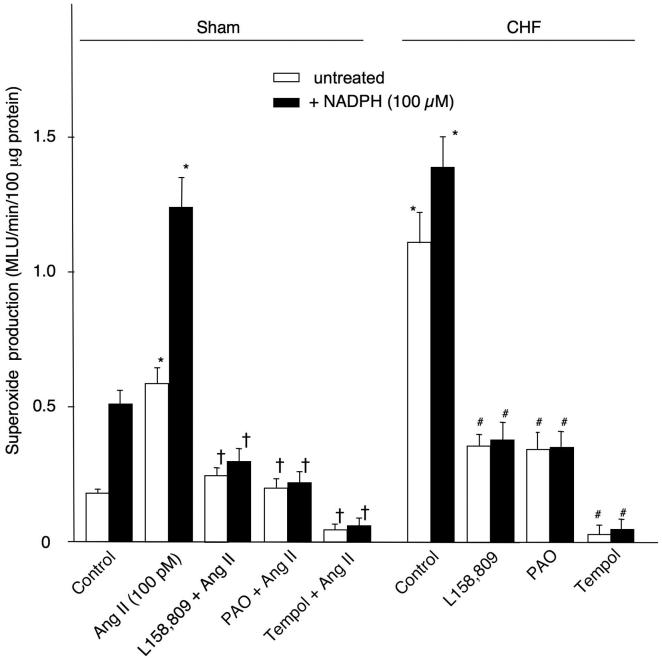
We also investigated the effects of L158,809, PAO, and tempol on the superoxide anion production in the CB homogenates from sham and CHF rabbits using lucigenin chemiluminescence method. In CBs from sham rabbits, a low level of superoxide anion production was detected (Fig. 6). Addition of NADPH (100 μM) to the CB homogenate significantly increased the superoxide anion production. Ang II (30 min, 100 pM) induced an elevation of the superoxide anion production in both untreated and NADPH treated CBs (p<0.05 vs control). L158,809, PAO, and tempol each inhibited the effect of Ang II on the superoxide anion production. In CBs from CHF rabbits, the superoxide anion production in the CB homogenates was elevated in both untreated and NADPH treated conditions, compared with that in sham rabbits. L158,809, PAO, and tempol each decreased superoxide anion production in CB tissues from CHF rabbits (Fig. 6). APO (100 μM) also attenuated the Ang II- and CHF-enhanced the superoxide anion production (n=5, data not shown).
Figure 6.
Superoxide anion production in CB homogenates from sham and CHF rabbits, as measured by lucigenin chemiluminescence. MLU: mean light units. Data are means ± SEM, n=7 in each group. *P<0.05 vs control in sham rabbits; †p<0.05 vs Ang II; #p<0.05 vs control in CHF rabbits.
4. Discussion
The results from the present study indicate that: (1) in sham rabbits, Ang II perfusion to the isolated CB preparation enhances the CB chemoreceptor sensitivity to hypoxia, and L158,809 (a selective AT1 receptor antagonist), PAO and APO (NADPH oxidase inhibitors), and tempol (a SOD mimetic) significantly blunts the Ang II-enhanced CB chemoreceptor sensitivity to hypoxia; (2) similarly, L158,809, PAO, APO, and tempol also decrease CB chemoreceptor responses to hypoxia in CHF rabbits; (3) mRNA and protein expressions of NADPH oxidase components (gp91phox, p47phox and p40phox) in the CB are markedly increased in CHF rabbits, compared to that in sham rabbits; (4) L158,809, PAO, APO, and tempol effectively attenuate Ang II- and CHF-induced superoxide anion production in the CB homogenates. These data suggest that an endogenous Ang II-NADPH oxidase-superoxide signaling pathway in the CB mediates the enhanced CB chemoreceptor activity to hypoxia in CHF.
Sympathetic hyperactivity in CHF contributes to late-stage deterioration of cardiac function [2-3]. Previous studies have shown that an enhanced peripheral chemoreflex sensitivity contributes significantly to the generalized sympathetic over activation in CHF patients and experimental animals [4, 5, 32, 33]. The CB is the primary sensor site of the peripheral chemoreflex. Our recent study revealed that elevation of endogenous Ang II and upregulation of AT1 receptors in the CB contributed to the enhanced CB chemoreceptor sensitivity to hypoxia and to the enhanced peripheral chemoreflex function in CHF rabbits [13]. However, the mechanism by which Ang II augments the CB chemoreceptor sensitivity to hypoxia was not known prior to this study.
Ang II activates NADPH oxidase [14], which serves as a significant source of intracellular reactive oxygen species (ROS) in many tissues [15-20]. Gao, et al. found that Ang II-NADPH oxidase-derived radical stress in autonomic areas of the brain was involved in the sympathoexcitation in rabbits with pacing-induced CHF [20], the same animal model as in the present study. Furthermore, a significant body of evidence indicates that the components of NADPH oxidase are present in the peripheral chemoreceptors including pulmonary neuroepithelial bodies and CB [21-23].
Our present results confirmed our previous study showing that endogenous Ang II and the AT1 receptor in the CB plays a major role in enhancing CB chemoreceptor sensitivity to hypoxia in CHF rabbits [13]. We further found that the Ang II- and CHF-enhanced CB chemoreceptor sensitivity to hypoxia is blunted by NADPH oxidase inhibitors (PAO and APO) and superoxide scavenger (tempol) (Figs. 1 and 2). At the same time, mRNA and protein expressions of NADPH oxidase components (gp91phox, p47phox and p40phox) and superoxide anion production are elevated in the CB from CHF rabbits (Figs. 3-6). An AT1 receptor antagonist, NADPH oxidase inhibitors, and superoxide scavenger attenuate the superoxide anion production induced by Ang II and CHF (Figs. 5 and 6). These results clearly indicate that Ang II-NADPH oxidase-derived superoxide anion signaling contributes to the enhanced CB chemoreceptor sensitivity to hypoxia in CHF rabbits.
The present study also confirms our previous observation [13] that neither Ang II nor L-158,809 affects CB chemoreceptor activity under normoxic conditions in sham and CHF rabbits, respectively. Consistent with this, the present study demonstrates that perfusion of the CB with either a NADPH oxidase inhibitor (PAO or APO) or tempol does not affect the baseline CB chemoreceptor activity in either sham or CHF rabbits (Fig. 2). These results, taken together, suggest that NADPH oxidase-derived superoxide anion signaling pathway mediates the Ang II-enhanced sensitivity of CB chemoreceptors to hypoxia in CHF, but does not contribute to the elevated basal chemoreceptor activity observed under normoxic conditions in our CHF animals.
In previous studies [6, 24], we have shown that downregulation of nNOS expression with decreased nitric oxide (NO) production in the CB is responsible for the elevated resting CB chemoreceptor discharge under normoxic conditions in CHF rabbits. The two constitutive isomers of NO synthase (NOS), neuronal NOS (nNOS) and endothelial NOS (eNOS), are localized to nerve fibers and vascular endothelium in the CB, and NO is inhibitory to the CB chemoreceptor activity [34]. We have also found that the NO donor, Snitroso-N-acetylpenicillamine or gene transfer of nNOS using an adenoviral vector to the CB in CHF rabbits reverses the enhanced CB chemoreceptor activity seen in the CHF state [6, 24]. Our present observation that NADPH oxidase-derived superoxide anion mediates the enhanced CB chemoreceptor sensitivity to hypoxia in CHF rabbits raises the question whether there may be an important interaction between these two signaling pathways (upregulation of Ang II-NADPH oxidase-superoxide anion pathway and downregulation of NOS-NO pathway) in CHF. Ang II may contribute to depressed bioavailable NO in the CB by suppressing nNOS gene expression [35] and/or increased scavenging of NO through superoxide anion production. Conversely, the downregulation of NO production in the CB in CHF may act to enhance the effects of Ang II by reduced scavenging of superoxide anion by NO. The relationship among NO, Ang II and superoxide anion on CB chemoreceptor function is not yet clear and deserves further study. But it is clear that upregulation of Ang II-NADPH oxidase-superoxide anion pathway and downregulation of NOS-NO pathway in the CB in CHF state have complementary effects on the CB chemoreceptor activity.
Our previous study has shown that endogenous Ang II modulates the CB chemoreceptor sensitivity to hypoxia via its inhibitory effects on O2-sensitive K+ channels in CB glomus cells from CHF rabbits [25]. In the present study, NADPH oxidase-derived superoxide anion mediated the Ang II- and CHF-induced enhancement of the CB chemoreceptor sensitivity to hypoxia. Therefore, It is possible that NADPH oxidase-derived superoxide anion is also involved in the inhibitory effect of Ang II on O2-sensitive K+ channels in CB glomus cells from CHF rabbits. Further studies are needed to define the involvement of NADPH oxidase-derived superoxide anion on the modulation of the O2-sensitive K+ channels in CB glomus cells from CHF rabbits.
NADPH oxidase is a complex enzyme consisting of two membrane-bound components (gp91phox and p22phox) and three cytosolic components (p67phox, p47phox, and p40phox) [29], which was first found in phagocytic cells [30]. Studies have shown that gp91phox, p22phox, p67phox, and p47phox subunits of the NADPH oxidase exist in the CB glomus cells in rats [21-22]. However, we found the mRNA of gp91phox, p47phox, and p40phox but not p22phox and p67phox are expressed in the rabbit CBs (Fig. 3) although we could detect the mRNA expression of p67phox and p22phoxin liver and kidney from sham rabbits as a positive control. Similarly, Gao, et al. also found that p22phox cannot be detected in the rabbit rostral ventrolateral medulla (RVLM) neurons [20]. Our present study cannot explain this discrepancy. One possibility is that p22phox and p67phox in the CB of rabbits may be unique homologues of the proteins that are undetectable using standard primers and antibodies. By analogy, recent expansion of information available in genome databases has led to identification of several novel homologues of gp91phox in animals, which forms the Nox family [36].
In the present study, superoxide anion production in the CB was increased in CHF rabbits compared to sham rabbits. Exogenous Ang II similarly enhanced the superoxide anion in the CB from sham rabbits (Fig. 5 and 6). Conversely, L158,809 and PAO markedly reversed the elevated superoxide anion production induced by Ang II and CHF to levels observed at rest in sham rabbits (Fig. 5 and 6). These results indicate that Ang II-NADPH oxidase is a main source of the elevated superoxide anion production in the CB in CHF. It also appears that superoxide anion production in the CB is limited by the availability of cofactor NADPH under normal (sham) conditions, but to a much smaller extent in the CHF condition. Thus both increased NADPH oxidase expression and increased NADPH availability play a role in the enhanced superoxide production in the CB in CHF.
We also found that the superoxide scavenger, tempol, nearly abolished superoxide anion production in CBs from both sham and CHF animals (Fig. 6), whereas L-158,809 or PAO decreased elevated Ang II and CHF induced superoxide levels to the control level observed in sham rabbits. Therefore, it seems that a small level of superoxide anion production arises from other sources (perhaps xanthine-xanthine oxidase, mitochondrial complex enzymes, etc) in the CB at rest in sham rabbits, and that this level of alternatively derived superoxide production is not altered by Ang II or by the induction of CHF (i.e. the level of superoxide production after NADPH oxidase inhibition was the same in CBs from sham and CHF rabbits and was comparable to the control level in sham rabbits, Fig. 6).
To what extent does superoxide production apart from NADPH oxidase activity influence CB chemoreceptor sensitivity in CHF? Although the inhibitory effect of tempol on superoxide anion production was greater than that of either L158,809 or PAO in CHF rabbits (Fig. 6), there was no difference among these three inhibitors on their ability to inhibit enhanced CB chemoreceptor activity in the CHF rabbits (Fig. 2). From these results, we assume that the superoxide anions produced by the Ang II-NADPH oxidase pathway, but not other superoxide ion sources, largely mediate the enhancement of the CB chemoreceptor activity in CHF rabbits.
Although our present study indicates that the Ang II-NADPH oxidase-superoxide anion signaling pathway mediates the enhanced peripheral chemoreceptor sensitivity in pacing-induced CHF rabbits, this signaling pathway has not been tested or confirmed in CHF patients with exaggerated peripheral chemoreflex function. In addition, the present study was performed in the isolated and perfused CB preparations, which could directly and indirectly affect the characteristics of the CB chemoreceptor in response to hypoxia. Therefore, care should be taken when extrapolating the data obtained here to the clinical phenomena observed in the CHF patients.
In conclusion, the expression of NADPH oxidase and the production of superoxide anion are elevated in the CBs from rabbits with CHF. This Ang II-NADPH oxidase-superoxide anion signaling pathway is involved in the enhanced CB chemoreceptor sensitivity to hypoxia in the CHF state.
Acknowledgements
The authors wish to thank Lisa Rasmussen, Kaye Talbitzer for their technical assistance, and Dr. Kurtis Cornish for his surgical assistance. This study was supported by a Program Project Grant from the Heart, Lung and Blood Institute of NIH (PO1-HL62222).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Seravalle G, Giannattasio C, Bossi M, Preti L, Cattaneo BM, et al. Reflex cardiovascular control in congestive heart failure. Am J Cardiol. 1992;69:17G–22G. doi: 10.1016/0002-9149(92)91251-x. [DOI] [PubMed] [Google Scholar]
- 3.Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol. 1995;18:3–8. doi: 10.1002/clc.4960181303. [DOI] [PubMed] [Google Scholar]
- 4.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999;86:1264–72. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–9. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 6.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol. 1999;86:1273–82. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- 7.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–70. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 8.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102:1854–62. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 9.Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–7. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 10.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and – independent pathways. Cardiovasc Res. 2003;60:315–25. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 11.van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, et al. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–72. doi: 10.1016/j.ijcard.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Allen AM. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J Physiol (Lond) 1998;510:773–81. doi: 10.1111/j.1469-7793.1998.773bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, et al. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71:129–38. doi: 10.1016/j.cardiores.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res. 2002;35:1001–15. doi: 10.1590/s0100-879x2002000900001. [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 16.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 17.Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am J Physiol. 2000;279:H2234–40. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 18.Franco Mdo C, Akamine EH, Di Marco GS, Casarini DE, Fortes ZB, Tostes RC, et al. NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: involvement of the renin-angiotensin system. Cardiovasc Res. 2003;59:767–75. doi: 10.1016/s0008-6363(03)00461-9. [DOI] [PubMed] [Google Scholar]
- 19.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–9. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 20.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–44. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 21.Kummer W, Acker H. Immunohistochemical demonstration of four subunits of neutrophil NAD(P)H oxidase in type I cells of carotid body. J Appl Physiol. 1995;78:1904–9. doi: 10.1152/jappl.1995.78.5.1904. [DOI] [PubMed] [Google Scholar]
- 22.Youngson C, Nurse C, Yeger H, Curnutte JT, Vollmer C, Wong V, et al. Immunocytochemical localization of O2-sensing protein (NADPH oxidase) in chemoreceptor cells. Microsc Res Tech. 1997;37:101–6. doi: 10.1002/(SICI)1097-0029(19970401)37:1<101::AID-JEMT10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Dvorakova M, Hohler B, Vollerthun R, Fischbach T, Kummer W. Macrophages: a major source of cytochrome b558 in the rat carotid body. Brain Res. 2000;852:349–54. doi: 10.1016/s0006-8993(99)02156-3. [DOI] [PubMed] [Google Scholar]
- 24.Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, et al. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res. 2005;97:260–7. doi: 10.1161/01.RES.0000175722.21555.55. [DOI] [PubMed] [Google Scholar]
- 25.LI YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol. 2006;575:215–27. doi: 10.1113/jphysiol.2006.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li JM, Shah AM. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res. 2001;52:477–86. doi: 10.1016/s0008-6363(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 27.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–60. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 28.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 29.Deleo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677–91. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 30.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–4. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 31.Kameda Y, Amanoo T, Tagawa T. Distribution and ontogeny of chromogranin A and tyrosine hydroxylase in the carotid body and glomus cells located in the wall of the common carotid artery and its branches in the chicken. Histochemistry. 1990;94:609–16. doi: 10.1007/BF00271988. [DOI] [PubMed] [Google Scholar]
- 32.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–7. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, et al. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur Heart J. 1997;18:480–6. doi: 10.1093/oxfordjournals.eurheartj.a015269. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respir Physiol. 1999;115:161–8. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 35.Kihara M, Umemura S, Kadota T, Yabana M, Tamura K, Nyuui N, et al. The neuronal isoform of constitutive nitric oxide synthase is up-regulated in the macula densa of angiotensinogen gene-knockout mice. Lab Invest. 1997;76:285–94. [PubMed] [Google Scholar]
- 36.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox 3, Nox 4, and Nox 5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]