Abstract
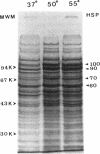
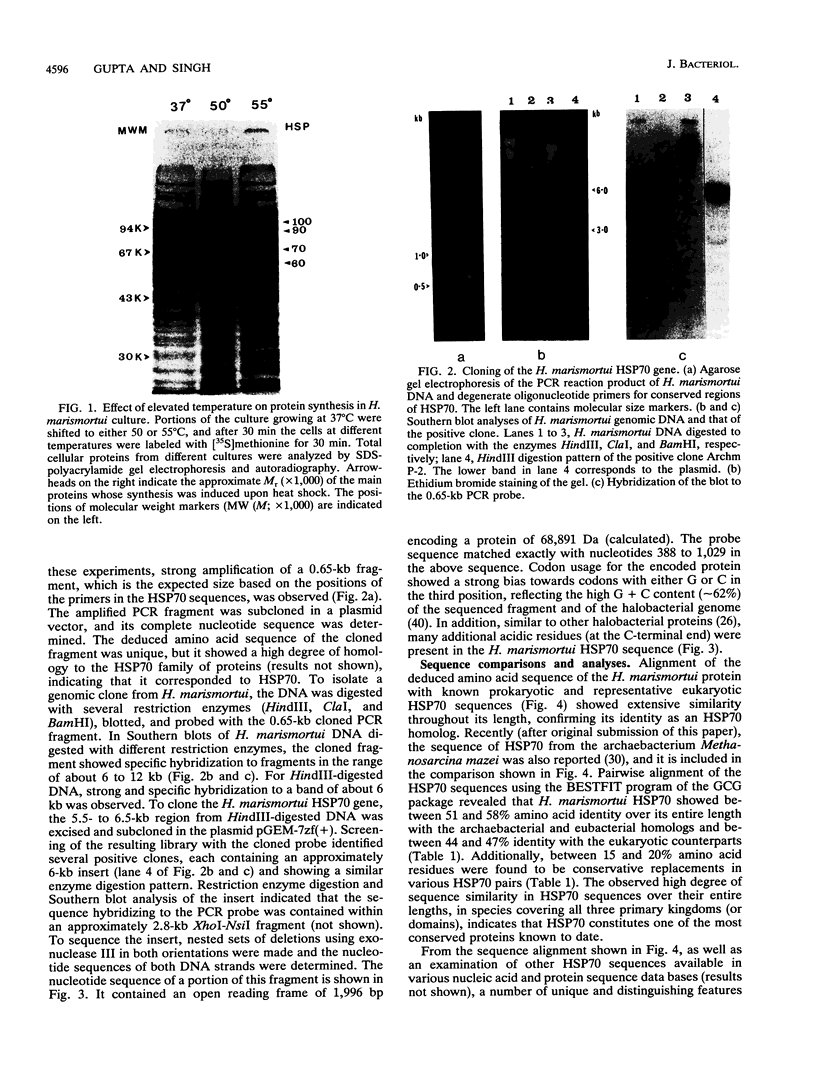
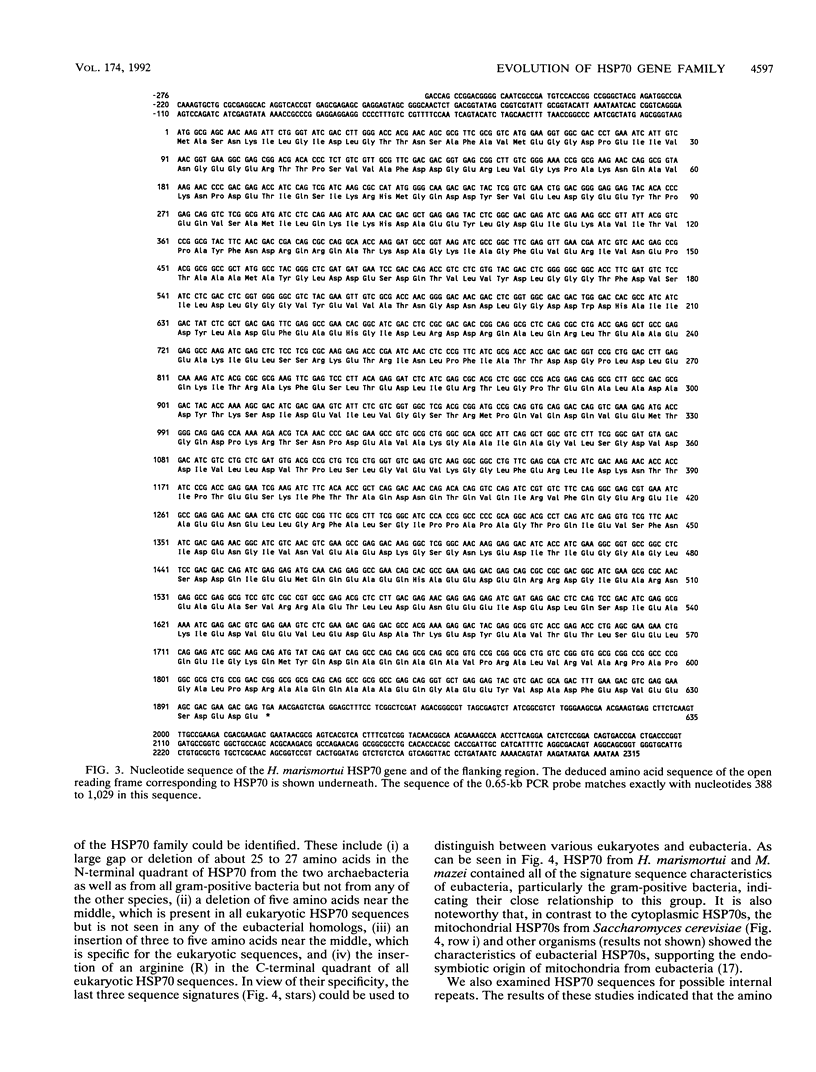
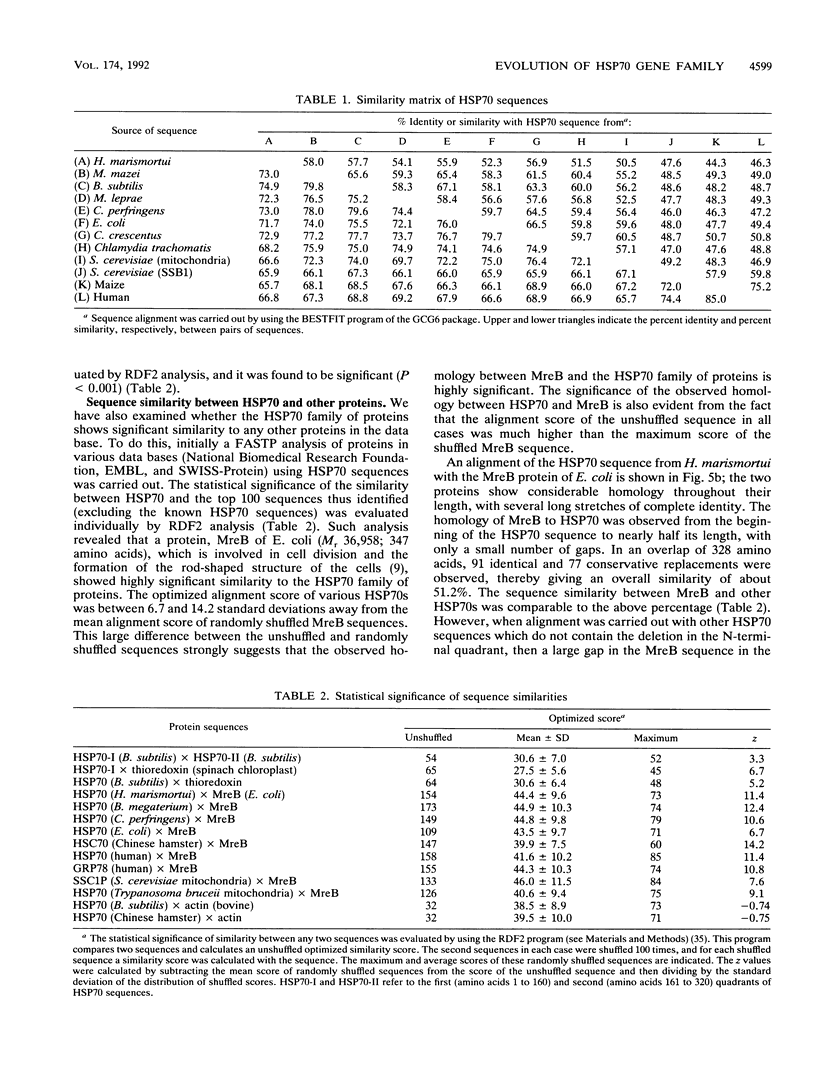
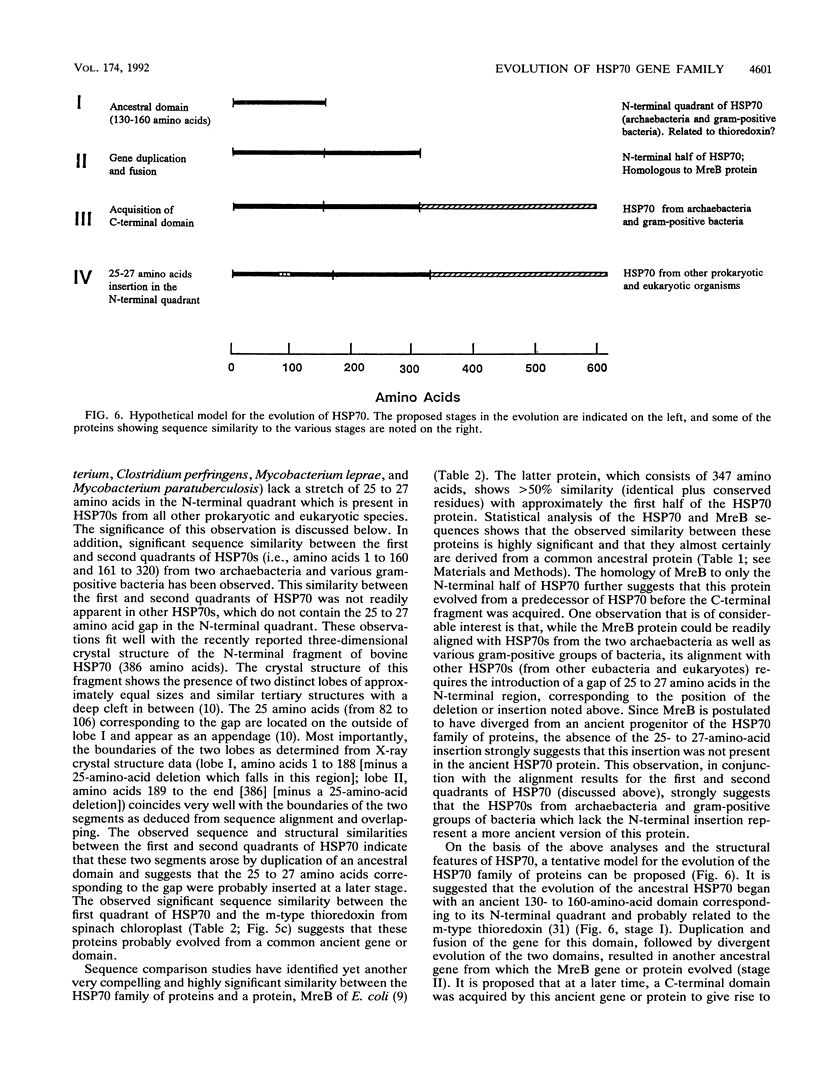
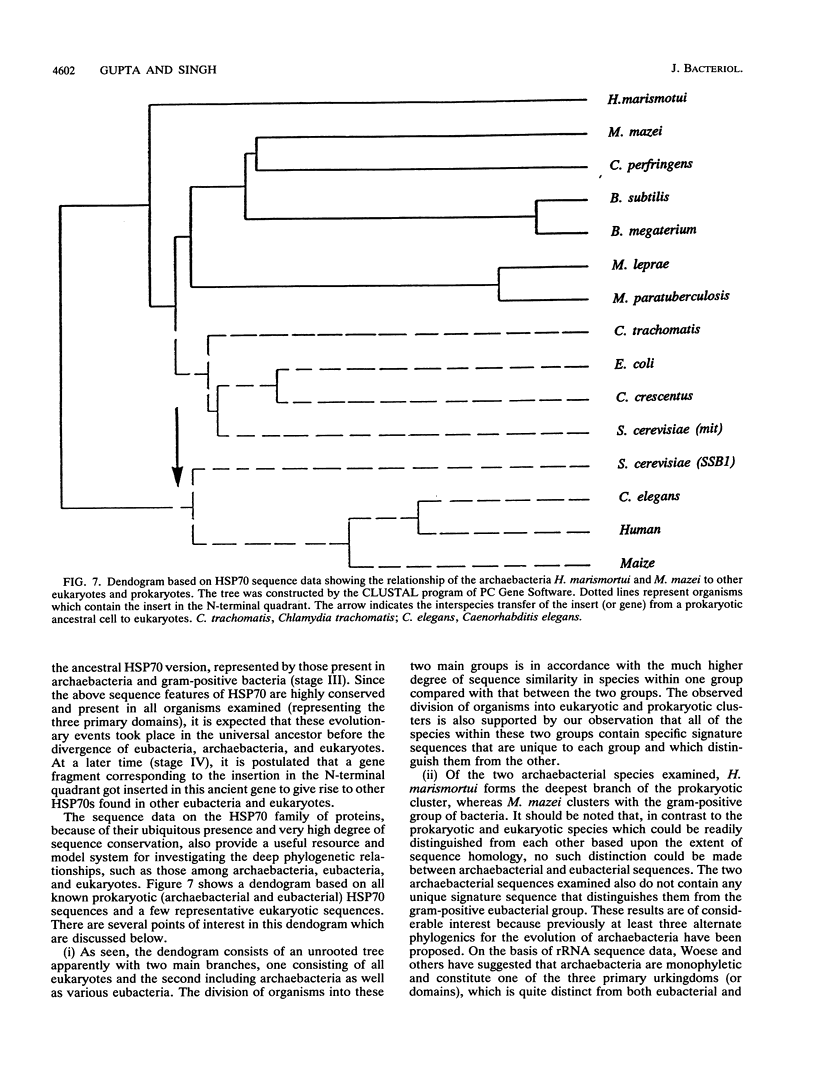
Heat shock induces the synthesis of a set of proteins in Halobacterium marismortui whose molecular sizes correspond to the known major heat shock proteins. By using the polymerase chain reaction and degenerate oligonucleotide primers for conserved regions of the 70-kDa heat shock protein (HSP70) family, we have successfully cloned and sequenced a gene fragment containing the entire coding sequence for HSP70 from H. marismortui. HSP70 from H. marismortui shows between 44 and 47% amino acid identity with various eukaryotic HSP70s and between 51 and 58% identity with its eubacterial and archaebacterial homologs. On the basis of a comparison of all available HSP70 sequences, we have identified a number of unique sequence signatures in this protein family that provide a clear distinction between eukaryotic organisms and prokaryotic organisms (archaebacteria and eubacteria). The archaebacterial (viz., H. marismortui and Methanosarcina mazei) HSP70s have been found to contain all of the signature sequences characteristic of eubacteria (particularly the gram-positive bacteria), which suggests a close evolutionary relationship between these groups. In addition, detailed analyses of HSP70 sequences that we have carried out have revealed a number of additional novel features of the HSP70 protein family. These include (i) the presence of an insertion of about 25 to 27 amino acids in the N-terminal quadrants of all known eukaryotic and prokaryotic HSP70s except those from archaebacteria and the gram-positive group of bacteria, (ii) significant sequence similarity in HSP70 regions comprising its first and second quadrants from organisms lacking the above insertion, (iii) highly significant similarity between a protein, MreB, of Escherichia coli and the N-terminal half of HSP70s, (iv) significant sequence similarity between the N-terminal quadrant of HSP70 (from gram-positive bacteria and archaebacteria) and the m-type thioredoxin of plant chloroplasts. To account for these and other observations, a model for the evolution of HSP70 proteins involving gene duplication is proposed. The model proposes that HSP70 from archaebacteria (H. marismortui and M. mazei) and the gram-positive group of bacteria constitutes the ancestral form of the protein and that all other HSP70s (viz., other eubacteria as well as eukaryotes) containing the insert have evolved from this ancient protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Ahuja R., Venner T. J., Gupta R. S. Identification of a protein altered in mutants resistant to microtubule inhibitors as a member of the major heat shock protein (hsp70) family. Mol Cell Biol. 1990 Oct;10(10):5160–5165. doi: 10.1128/mcb.10.10.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Sprott G. D., Whippey P. Ultrastructure, inferred porosity, and gram-staining character of Methanospirillum hungatei filament termini describe a unique cell permeability for this archaeobacterium. J Bacteriol. 1991 Jan;173(1):130–140. doi: 10.1128/jb.173.1.130-140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. The 75-kilodalton cytoplasmic Chlamydia trachomatis L2 polypeptide is a DnaK-like protein. Infect Immun. 1990 Jul;58(7):2098–2104. doi: 10.1128/iai.58.7.2098-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. The origin of eukaryotic and archaebacterial cells. Ann N Y Acad Sci. 1987;503:17–54. doi: 10.1111/j.1749-6632.1987.tb40596.x. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., McKee A. H., Doolittle W. F. Archaebacterial heat-shock proteins. EMBO J. 1984 Apr;3(4):745–749. doi: 10.1002/j.1460-2075.1984.tb01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M., Wachi M., Ishino F., Tomioka S., Ito M., Sakagami Y., Suzuki A., Matsuhashi M. Determinations of the DNA sequence of the mreB gene and of the gene products of the mre region that function in formation of the rod shape of Escherichia coli cells. J Bacteriol. 1988 Oct;170(10):4619–4624. doi: 10.1128/jb.170.10.4619-4624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., McKay D. B., Kabsch W., Holmes K. C. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5041–5045. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley K. A., Singh B., Gupta R. S. Cloning of HSP70 (dnaK) gene from Clostridium perfringens using a general polymerase chain reaction based approach. Biochim Biophys Acta. 1992 Mar 24;1130(2):203–208. doi: 10.1016/0167-4781(92)90529-9. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. L., Gober J. W., Shapiro L. Expression of the Caulobacter heat shock gene dnaK is developmentally controlled during growth at normal temperatures. J Bacteriol. 1990 Jun;172(6):3051–3059. doi: 10.1128/jb.172.6.3051-3059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Li W. H. Phylogenetic analysis based on rRNA sequences supports the archaebacterial rather than the eocyte tree. Nature. 1989 May 11;339(6220):145–147. doi: 10.1038/339145a0. [DOI] [PubMed] [Google Scholar]
- Gray M. W. The evolutionary origins of organelles. Trends Genet. 1989 Sep;5(9):294–299. doi: 10.1016/0168-9525(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Hearne C. M., Ellar D. J. Nucleotide sequence of a Bacillus subtilis gene homologous to the dnaK gene of Escherichia coli. Nucleic Acids Res. 1989 Oct 25;17(20):8373–8373. doi: 10.1093/nar/17.20.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert A. M., Kropinski A. M., Jarrell K. F. Heat shock response of the archaebacterium Methanococcus voltae. J Bacteriol. 1991 May;173(10):3224–3227. doi: 10.1128/jb.173.10.3224-3227.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann J. A. Genetics of gene transfer between species. Trends Genet. 1991 Jun;7(6):181–185. doi: 10.1016/0168-9525(91)90433-q. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Origin of the eukaryotic nucleus determined by rate-invariant analysis of rRNA sequences. Nature. 1988 Jan 14;331(6152):184–186. doi: 10.1038/331184a0. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Origin of the eukaryotic nucleus: eukaryotes and eocytes are genotypically related. Can J Microbiol. 1989 Jan;35(1):109–118. doi: 10.1139/m89-017. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Tracing origins with molecular sequences: metazoan and eukaryotic beginnings. Trends Biochem Sci. 1991 Feb;16(2):46–50. doi: 10.1016/0968-0004(91)90020-v. [DOI] [PubMed] [Google Scholar]
- Lam W. L., Cohen A., Tsouluhas D., Doolittle W. F. Genes for tryptophan biosynthesis in the archaebacterium Haloferax volcanii. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6614–6618. doi: 10.1073/pnas.87.17.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Löhlein-Werhahn G., Goepfert P., Eggerer H. Purification and properties of an archaebacterial enzyme: citrate synthase from Sulfolobus solfataricus. Biol Chem Hoppe Seyler. 1988 Feb;369(2):109–113. doi: 10.1515/bchm3.1988.369.1.109. [DOI] [PubMed] [Google Scholar]
- Macario A. J., Dugan C. B., Conway de Macario E. A dnaK homolog in the archaebacterium Methanosarcina mazei S6. Gene. 1991 Dec 1;108(1):133–137. doi: 10.1016/0378-1119(91)90498-z. [DOI] [PubMed] [Google Scholar]
- Maeda K., Tsugita A., Dalzoppo D., Vilbois F., Schürmann P. Further characterization and amino acid sequence of m-type thioredoxins from spinach chloroplasts. Eur J Biochem. 1986 Jan 2;154(1):197–203. doi: 10.1111/j.1432-1033.1986.tb09379.x. [DOI] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Mevarech M., Hirsch-Twizer S., Goldman S., Yakobson E., Eisenberg H., Dennis P. P. Isolation and characterization of the rRNA gene clusters of Halobacterium marismortui. J Bacteriol. 1989 Jun;171(6):3479–3485. doi: 10.1128/jb.171.6.3479-3485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Pühler G., Leffers H., Gropp F., Palm P., Klenk H. P., Lottspeich F., Garrett R. A., Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. doi: 10.1073/pnas.86.12.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. The SSB1 heat shock cognate gene of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jun 26;17(12):4891–4891. doi: 10.1093/nar/17.12.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K., Inglis N. F., Rae B., Donachie W., Sharp J. M. Complete nucleotide sequence of a gene encoding the 70 kd heat shock protein of Mycobacterium paratuberculosis. Nucleic Acids Res. 1991 Aug 25;19(16):4552–4552. doi: 10.1093/nar/19.16.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. D., Setlow P. Nucleotide sequence of a Bacillus megaterium gene homologous to the dnaK gene of Escherichia coli. Nucleic Acids Res. 1987 May 11;15(9):3923–3923. doi: 10.1093/nar/15.9.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Olsen G. J. Archaebacterial phylogeny: perspectives on the urkingdoms. Syst Appl Microbiol. 1986;7:161–177. doi: 10.1016/s0723-2020(86)80001-7. [DOI] [PubMed] [Google Scholar]