Abstract
The vaccinia virus temperature-sensitive mutations Cts6 and Cts9 were mapped by marker rescue and DNA sequencing to the A28 gene. Cts6 and Cts9 contain an identical 2-bp deletion truncating the A28 protein and removing the fourth conserved cysteine near the C-terminus. Cts9 mutant virions produced at 40°C were non-infectious and unable to cause cytopathic effect. However, the mutant A28 protein localized to purified mature virions (MV) at 31°C and 40°C. MV of Cts9 produced at 40°C bound to cells but did not enter cells. Low pH treatment of Cts9-infected cells at 18h p.i. failed to produce fusion from within at 40°C, but gave fusion at 31°C. Adsorption of Cts9 mutant virions to cells followed by low pH treatment showed a defect in fusion from without. The Cts9 phenotype suggests that the A28 protein is involved in both virus entry and cell-cell fusion, and supports the linkage between the two processes.
Keywords: Poxvirus, Vaccinia virus, Virus binding and entry, Cell-cell fusion, Temperature-sensitive mutants, Fusion from within, Fusion from without
INTRODUCTION
The poxviruses are a large and diverse group of DNA viruses that replicate in the cytoplasm of infected cells (Moss, 2001). Many aspects of their life cycle, including DNA replication, transcription, and immune evasion, are relatively well understood, and significant progress has been made in unraveling the complex process of virion morphogenesis (reviewed in (Condit et al., 2006) and (Smith et al., 2002). However, until recently little was known about the process by which poxvirus particles gain access to the cytoplasm of susceptible cells.
Vaccinia virus (VV) is the best-studied poxvirus, and produces two major forms of infectious virus: an intracellular form, MV, (mature virion) which is surrounded by a single lipid bilayer membrane, and an extracellular form, EV, (extracellular virion), which is surrounded by two lipid bilayer membranes. During morphogenesis MV is wrapped with a Golgi-derived double membrane to produce a wrapped form, WV, (wrapped virion) covered with three membrane layers. During fusion of WV with the cell membrane, the outermost envelope is lost, generating EV that is either released from infected cells or remains attached at the cell surface.
Binding of MV to the cell surface is thought to be mediated at least partly by three virion proteins that bind to glycosaminoglycans, namely D8, which binds chondroitin sulfate (Hsiao et al., 1999), and the heparan sulfate-binding proteins A27 (Chung et al., 1998) and H3 (Lin et al., 2000). However, none of these proteins is essential for virus attachment, and studies with soluble glycosaminoglycans and polyanions have shown either relatively modest reductions in infection, or no effect, depending on the cell line (Carter et al., 2005).
Entry of MV into cells is thought to occur by fusion between the membrane of MV and the cell membrane, either directly at the cell surface (Carter et al., 2005) or following internalization into acidic endosomes (Townsley et al., 2006). Both pathways may occur on infection of the same host cells, and the net effect is release of virus cores into the cytoplasm, followed by uncoating and early transcription. Based on electron microscopy, the EV form sheds its outer membrane by a non-fusogenic mechanism at the cell surface, and the released MV underneath the broken EV membrane or “shroud” then fuses with the plasma membrane (Law et al., 2006). Thus, although the EV and MV particles may interact with different cellular attachment factors (Vanderplasschen et al., 1998), the entry pathways converge at the step of MV fusion with the cell membrane. Cell-cell fusion mediated by EV at the surface of infected cells (fusion from within) or by MV added exogenously to cells (fusion from without) is triggered by low pH treatment (Gong et al., 1990), and probably reflects the natural process of virus fusion to cell membranes within endosomes.
Temperature-sensitive mutants of VV have been useful in defining roles for essential genes in virus morphogenesis, as have inducible mutants in which a specific gene is only expressed in the presence of an inducer. Several conserved MV surface membrane proteins have recently been shown to be required for the entry of virus particles into the cytoplasm, including A28 (Senkevich et al., 2004b), H2 (Senkevich & Moss, 2005), A21 (Townsley et al., 2005b), L5 (Townsley et al., 2005a), A16 (Ojeda et al., 2006b), G3 (Izmailyan et al., 2006), and G9 (Ojeda et al., 2006a). Inducible mutants for any one of these seven genes have the same phenotype, and are defective in virus entry and in causing fusion from within and fusion from without. In the absence of any one of these proteins, normal virion assembly occurs, and binding of MV to cells is unaffected. The seven proteins together with J5 have been reported to associate in an “entry-fusion” complex (Senkevich et al., 2005). In addition the VV F9 protein is required for virus entry, and interacts with proteins of the complex (Brown et al., 2006).
Orthologs of the vaccinia A28 protein are found in poxviruses belonging to both the Entomopoxvirinae and Chordopoxvirinae subfamilies, and in all cases there is complete conservation of the four cysteine residues which are known in the VV WR A28 protein to participate in two intramolecular disulfide bonds formed by the vaccinia-encoded redox pathway (Senkevich et al., 2002; Senkevich et al., 2004a). Our studies with a temperature-sensitive VV mutant in the A28 protein show that deletion of 14 amino acids from the A28 C-terminus, including the fourth conserved cysteine residue, surprisingly results in a virus that is viable at 31°C. However, at 40°C the A28 ts mutant synthesizes mature virions that are unable to enter cells, and are defective in mediating cell-cell fusion following low pH treatment.
RESULTS
Mapping of the mutations Cts6 and Cts9 to the A28 gene region by marker rescue
The temperature sensitive vaccinia virus mutants Cts6 and Cts9 comprise a complementation group that has a “normal” phenotype, that is, they display wild type protein and DNA synthesis at the non-permissive temperature of 40°C (Condit & Motyczka, 1981). Recombination between the two mutants was undetectable, suggesting that the mutations lie close together (Condit & Motyczka, 1981). Using a library of overlapping cosmid clones, Thompson and Condit (1986) showed that Cts6 mapped to an approximately 3 kb region of DNA located in the middle of the HindIII A fragment of the vaccinia genome. Further preliminary mapping (not shown) using 5 kb PCR products spanning this region (Luttge & Moyer, 2005) showed that the mutation was apparently within a DNA fragment LM32 comprising genes A25.3L (part of the fragmented ATI gene) through A29L (Fig. 1).
Figure 1. Marker rescue of Cts6.
At the top is a diagram of a 7 kb region of the vaccinia genome within which Cts6 has been previously mapped, including genes A25L through A29L. Genes are represented by arrows, with the gene name and protein function indicated above. The horizontal lines immediately below represent a subset of PCR products used in the rescue of Cts6. The gene(s) included in each PCR product are indicated to the right of the product; LM32 corresponds to a 5 kb PCR product used in preliminary studies. The bottom of the figure shows selected crystal violet stained 60-mm dishes resulting from the marker rescue, with the corresponding PCR product labeled below the dish. Note that within the region comprising the non-essential genes A25L and A26L, the Copenhagen DNA sequence, from which the familiar gene nomenclature is derived, differs significantly from the WR sequence. In WR, the A type inclusion (ATI) gene is fragmented into 4 ORFs which we have called A25L, A25.1L, A25.2L and A25.3L. These 4 ORFs correspond to VACWR145, VACWR146, VACWR147 and VACWR148 respectively in the Poxvirus Bioinformatics Resource Center database (www.poxvirus.org). In the Copenhagen sequence, the A26L ORF is formed by a large deletion which spans A25.2 and A25.3 and fuses the N-terminus of the p4c gene with sequence near the 3' end of the ATI gene corresponding to the 3' end of the A25.1L ORF. The A25L ORF in WR and Copenhagen are approximately equivalent.
Initial attempts at fine mapping the mutations using PCR products corresponding precisely to individual open reading frames within this region did not result in a significant rescue (data not shown). We therefore attempted marker rescue using PCR products that incorporated two or more adjacent genes, specifically A25L-A26L, A25L-A27L, A27L-A28L, A27L-A29L, and A28L-A29L. Fig. 1 shows a subset of the results demonstrating that rescue of Cts6 did not occur with products containing A25L-A27L, A28L-A29L, or with A27L or A28L alone, however rescue was observed with PCR DNA containing both A27L and A28L. Similar results were obtained with Cts9 (not shown). This result suggests that the mutations map near the 5' end of A27L or at the 3' end of A28L.
Cts6 and Cts9 contain an identical mutation resulting in truncation of the A28 protein prior to the fourth conserved cysteine residue
To determine precisely the mutations that cause the temperature sensitivity in Cts6 and Cts9, the A27L and A28L genes from both viruses were sequenced. The A27L gene from both viruses was wild type in DNA sequence, however both mutants contained a two nucleotide deletion near the 3' end of the A28L gene (Fig. 2A), consistent with the marker rescue results. The observation that the mutations in the two viruses are identical is in agreement with the fact that recombination between the two mutants was undetectable (Condit & Motyczka, 1981). The mutants were isolated at different times using a hydroxylamine mutagenesis protocol, which should cause nucleotide substitutions rather than deletions (Condit & Motyczka, 1981). We speculate that the mutants were in fact pre-existing siblings present in the stock of virus used in the mutagenesis.
Figure 2. Sequences of the A28 gene and protein from wild type VV and from Cts6 and Cts9.
A) Nucleotide alignment of the 3' end of the A28L gene from wild type, Cts6, and Cts9 viruses. In Cts6 and Cts9 cytosine residues located at positions 394 and 395 are deleted, which are indicated by ^ ^ below the sequence.
B) Amino acid sequence alignment of A28 protein from wild type, Cts6, and Cts9. The first 131 amino acids are identical in mutant and wild type viruses. The deletion of the two adjacent C residues causes a frameshift which results in three amino acid substitutions (indicated by * under the sequence) followed by a premature stop codon causing truncation. Conserved cysteine residues are indicated by “C” in boldface. The A28 protein in our laboratory isolate of vaccinia WR was found to contain asparagine at position 124 rather than the aspartic acid expected from the published sequence (Senkevich et al., 2004a). A similar N124 substitution relative to the published VV WR sequence was found in the A28 protein of VV Copenhagen (Goebel et al., 1990) and of VV strain MVA.
C) A diagram of the wt and mutant A28 proteins. A transmembrane domain, indicated by a gray box and labeled TM, is located between the 4th and 26th amino acids. The open box at the C-terminal end of the mutant protein represents the three substitutions encoded by the mutant viruses, which begin at amino acid 132. The Cs inside the proteins represent conserved cysteines.
The deletion of two adjacent G residues causes a translation frameshift at codon 132 followed by a premature termination after two further codons, generating a C-terminal truncated protein 134 amino acids in length (Fig. 2B, C). The wild type A28 protein is 146 amino acids in length and contains five cysteine residues. The first cysteine, at position 17, lies within the predicted N-terminal transmembrane domain (Fig. 2C) and is not conserved in all poxvirus orthologs. The remaining four cysteine residues are completely conserved in all known A28 orthologs (Senkevich et al., 2004a), including those from the insect poxviruses Amsacta moorei entomopoxvirus and Melanoplus sanguinipes entomopoxvirus. Interestingly, the Cts6/Cts9 truncation deletes the fourth conserved cysteine at position 139 in the A28 protein, closest to the C-terminus.
Purified mature virions of Cts6 produced at both permissive and non-permissive temperatures contain the truncated A28 protein
Analysis of the defect in the Cts9 mutant in infected cells at the non-permissive temperature of 40°C by electron microscopy did not reveal any obvious abnormality. All of the stages of virion morphogenesis were observed at both 31°C and 40°C, and apparently normal mature virions were produced following infection by Cts9 at 40°C (data not shown). This finding was consistent with earlier observations that protein and DNA synthesis were not grossly perturbed in the mutant infection (Condit & Motyczka, 1981).
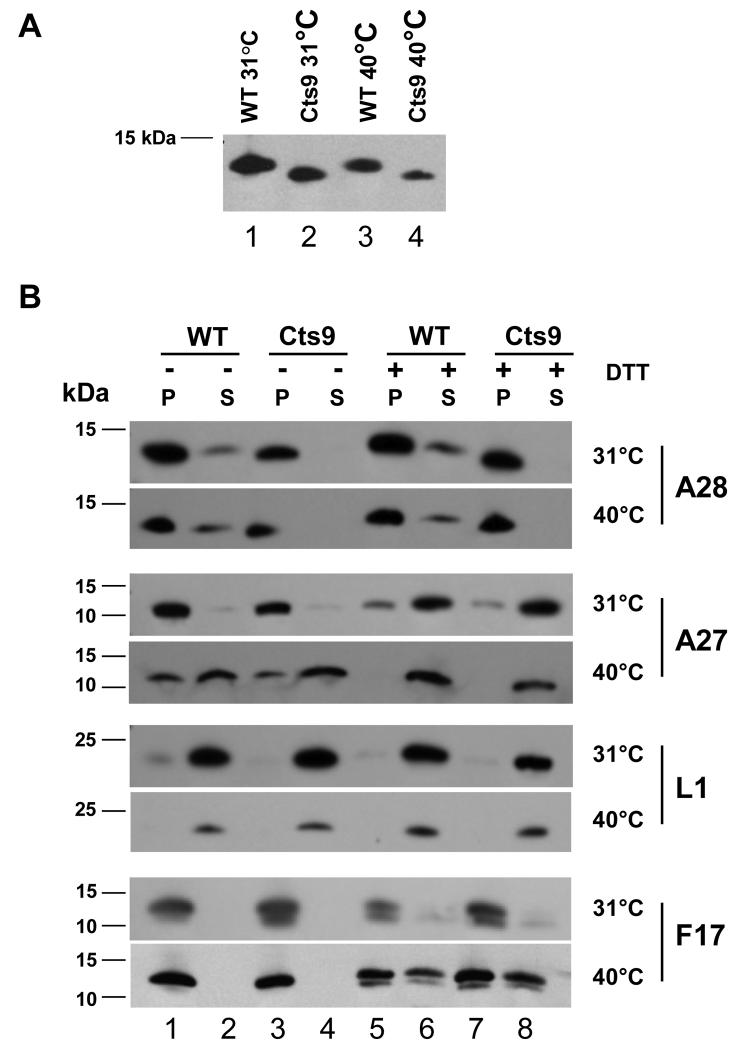
The presence of mutant A28 protein in the virions produced at both 31°C and 40°C was investigated by immunoblotting with a polyclonal antibody against a synthetic peptide spanning residues 56-75 of wild type A28 protein (Materials and Methods). The antibody enabled visualization of the wild-type A28 protein in purified MV of wild type virus (Fig. 3A). A similar band was not detected in extracts from uninfected cells (data not shown). A faster-migrating band relative to the wt A28 protein was detected in purified MV of Cts9 grown at either 31°C or at 40°C (Fig. 3A), indicating that the truncated A28 protein remained associated with virions synthesized at either temperature. The mobility difference between wt and Cts9 A28 protein was clearly visible following extended electrophoresis in a 10%-20% gradient Tris-Tricine gel (Fig. 3A) but was less evident following separation for shorter times (Fig. 3B).
Figure 3. Immunoblot analysis of purified wild type and mutant virions.
A. Different mobilities of A28 protein from WT and Cts9 virions. Virions were incubated in the presence of 50 mM Tris for 30 minutes at 37°C, pelleted in a microfuge for 30 minutes, and subjected to electrophoresis on a 10-20% acrylamide Tris-tricine gel before immunoblotting with anti-A28 antibody. The nature of the A28 allele (WT or Cts9) and the temperature at which the virions were produced are shown above each lane.
B. Western blot analysis of protein distribution in fractionated virions. Wild type (WT) or mutant (Cts9) virions grown at 31°C or 40°C were separated in the absence (−) or presence (+) of DTT into core (pellet, P) and membrane (supernatant, S) fractions and run on a 10-20% Tris-Tricine gel. Proteins were subjected to Western blot analysis with anti-A28, anti-A27, anti-L1, or anti-F17 antibody, as indicated on the right. Molecular weight markers are indicated on the left.
The partitioning of wt and Cts9 A28 protein into membrane (soluble) and core (insoluble) fractions was followed after treatment of virions with 1% NP-40 detergent in the absence or presence of the reducing agent DTT. The pellet and supernatant fractions were separately probed with antibodies against A28, A27, L1, and F17 (Fig. 3). The A28 protein has a predicted transmembrane domain from residues 4-26, and this region is unaltered in the Cts9 mutant. However, wt A28 protein did not partition as expected for a membrane protein, and was found predominantly in the insoluble fraction after treatment with both detergent and DTT (Fig. 3B). Senkevich et al. (2004a) also found that A28 was only partially solublized by NP40, and remained core associated after incubation of virions with both NP-40 and DTT. The Cts9 A28 protein behaved in a manner similar to wt A28. Traces of wt A28 protein were detected in the soluble fraction of wild type MV grown at 31°C or 40°C (Fig. 3B). The Cts9 protein appeared somewhat less abundant than the wt A28 protein, and was not detectable in the soluble fraction. The absence of the Cts9 protein from the soluble fraction may reflect either intrinsically reduced solubility of the mutant protein, or possibly altered interactions with other virion components.
The L1 protein behaved as a membrane protein as it was solubilized efficiently by treatment with 1% NP-40 in both the absence and presence of DTT (Fig. 3B). The core protein F17, previously known as VP11 (Kao & Bauer, 1987), was present exclusively in the insoluble fraction following treatment with detergent alone (Fig. 3B). A27 (sometimes called the 14kDa fusion protein), is a membrane-associated protein that interacts with the membrane protein A17 (Rodriguez et al., 1993), although A27 is not itself an integral membrane protein. We detected A27 in the both soluble and insoluble fractions following detergent treatment of wt or Cts9 virions grown at 40°C, but A27 was almost entirely soluble following detergent and DTT treatment (Fig. 3B). The proportion of A27 that was soluble from virions grown at 31°C also increased in the presence of DTT (Fig. 3B).
The synthesis of virions in the presence of truncated Cts9 A28 protein at either 31°C or 40°C did not alter the amounts or fraction distribution of A27, L1, or F17 in comparison with virions containing wt A28 protein produced at the same temperature (Fig. 3B), suggesting that Cts9 intracellular virions were not grossly perturbed in their assembly, even when assembled at 40°C. The biological properties of Cts9 mutant virions were assessed by determining their ability to yield plaques and cytopathic effect when added to cells.
Cts9 virus particles produced at 40°C have an elevated particle/pfu ratio, and are unable to produce cytopathic effect at 31°C
Mature virions were purified from BSC-40 cells infected with either wtVV or Cts9, at 31°C or 40°C, and the yield measured in terms of both particles (by optical density) and infectious virus (by plaque assay at 31°C). The Cts9 mutant produced a normal yield of virus particles per infected cell at 40°C of approximately 1,000 (Table 1), which was similar to the value of 1,067 obtained for wtVV grown at 40°C. Particle production by Cts9 at 31°C was also normal (Table 1), and similar to that seen for wtVV under comparable conditions. Cts9 particles from infection at 31°C had a particle/pfu ratio of 42 and were thus almost normal, although with a slightly higher value than for wtVV grown at 31°C (Table 1). In contrast, Cts9 particles from infection at 40°C had a particle/pfu ratio of 5,101, approximately 150-fold higher than the corresponding value for wtVV (Table 1).
Table 1.
Particle yield and particle/pfu ratio for WT and Cts9 viruses
| Virus | Growth temperature (°C) |
Yield (particles/cell) |
Particles/pfu |
|---|---|---|---|
| WT | 31 | 1467 | 14 |
| WT | 40 | 1067 | 34 |
| Cts9 | 31 | 1333 | 42 |
| Cts9 | 40 | 1000 | 5101 |
The ability of non-infectious particles to cause cytopathic effect was investigated. Confluent monolayers of BSC-40 cells were infected with purified MV from wild type VV grown at 31°C or 40°C, and from Cts9 grown at both temperatures. A multiplicity of 5 was used, with an equivalent number of particles for the Cts9 mutant grown at 40°C. The cells were incubated at 31°C , and cytopathic effect (CPE) was assessed following staining of cellular actin with phalloidin-AlexaFluor 568 at 6h p.i. Mock infected cells (Fig. 4A) showed no CPE, as expected. Purified MV of wtVV grown at 40°C gave extensive CPE, with cell shrinkage and rounding (Fig. 4B), as did wtVV virions produced at 31°C (not shown). Cts9 MV resulting from infection at 31°C induced CPE (Fig. 4C) similar to that seen with wild type MV. In contrast, Cts9 virions produced at 40°C were unable to cause detectable CPE under the conditions used here (Fig. 4D).
Figure 4. Lack of cytoplasmic effect following infection with Cts9 virions produced at the non-permissive temperature.

BSC-40 cells were mock-infected (A), or infected at 31°C with purified MV of wtVV grown at 40°C (B), with Cts9 virions grown at 31°C (C), or with Cts9 virions produced at 40°C (D). At 6h p.i., the cells were fixed and stained for actin with phalloidin-AlexFluor 568.
Mature virions of Cts9 generated at 40°C are able to bind to cells, but are unable to produce intracellular cores
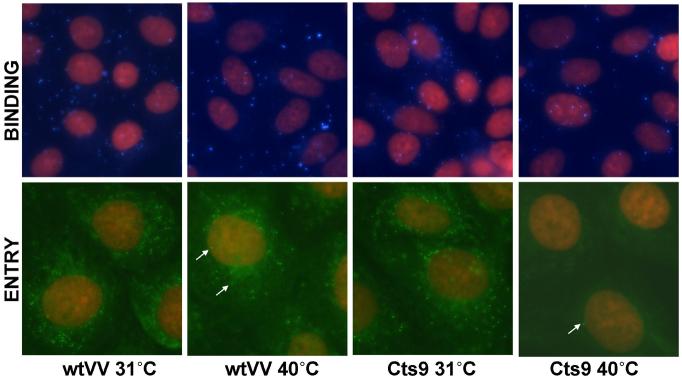
The inability of Cts9 virus particles produced at the non-permissive temperature to cause cytopathic effect suggested that they were defective in a very early step of the virus lifecycle, such as binding of virions to cells, entry into cells, or early transcription. An analysis of purified Cts9 virions grown at 40°C for ability to carry out transcription in vitro at 31°C indicated that these virions were not defective in transcription (data not shown), suggesting that the virions may be unable to bind to cells, or to enter into cells. We tested for binding of purified virions by incubating cells with virions at room temperature and then staining with monoclonal antibody against the A27 protein, an abundant component of IMV. The results (Fig. 5, top row) clearly indicated that Cts9 virions produced at either 31°C or 40°C were able to bind to cells. Entry of virions was followed by binding purified MV to cells at room temperature, transferring to 31°C to allow virus entry to occur, and then staining for intracellular virus cores with A4 antibody (Rodriguez et al., 1985). The core proteins do not become accessible to antibody until after uncoating, even with virion permeabilization (Vanderplasschen et al., 1998). Using this method, intracellular cores were readily detected following infection with wtVV grown at either temperature, and after infection with Cts9 virions produced at 31°C (Fig. 5, lower row). However, under the same conditions Cts9 MV produced at 40°C was almost entirely defective in generating intracellular cores, with only one or two cores at most visible per cell, in contrast with the abundant cores visible from the other infections (Fig. 5). The defect in non-infectious Cts9 virions therefore appeared to be specifically in between virus binding and the generation of intracellular cores, i.e. at the step of virus entry or uncoating. A defect in virus entry might be associated with alterations in cell-cell fusion mediated by cell-associated enveloped virus (fusion from within) or by bound MV (fusion from without). In contrast, mutation affecting uncoating would not be expected to alter the fusogenic properties of virus particles.
Figure 5. Binding of Cts9 virions to cells but lack of entry.
The upper four panels show binding to CV-1 cells of purified mature virions of either WT VV or Cts9 grown at 31°C or 40°C. CV-1 cells were infected at a multiplicity of 10 (for WT VV or Cts9 grown at 31°C), or for Cts9 grown at 40°C with an equivalent number of virus particles. Following adsorption of virions to cells for 1h at room temperature, the cells were fixed and reacted with anti-A27 mAb followed by goat anti-mouse FITC secondary Ab. The virions were pseudocolored cyan. Nuclei were counterstained by propidium iodide (red). The lower four panels show the result of the virus entry assay. Mature virions were adsorbed to cells at RT, and the cells transferred to 31°C for 2h to allow virus entry to occur. The cells were fixed, permeabilized, and stained with rabbit anti-A4 serum followed by goat anti-rabbit FITC (green) to visualize intracellular cores. Counterstaining of nuclei was with propidium iodide. The cells were photographed with a Zeiss Axiovert 200M inverted fluorescence microscope. Arrows in the lower panels indicate representative core particles.
The Cts9 mutant is unable to mediate fusion from within at 40°C, and is partially defective at 31°C
Cell-cell fusion is thought to reflect virus-cell fusion events that occur during the entry of virus into susceptible cells. We tested the Cts9 mutant for the ability to mediate fusion from within at 31°C and at 40°C. Cells were infected with either wtVV or Cts9 at a multiplicity of 5 at 31°C or 40°C, and treated briefly at 16h p.i. with buffer at pH 4.6. Low pH treatment resulted in extensive cell-cell fusion following infection with wtVV at either temperature that was readily observed by phase contrast microscopy (Fig. 6A). No fusion was observed after treatment of the same infected cells with buffer at pH 7.4 (Fig. 6A). The Cts9 mutant produced clearly visible cell-cell fusion at 31°C following low pH treatment, but no cell-cell fusion was observed at 40°C after acid treatment (Fig. 6A).
Figure 6. Fusion from within by Cts9, and effect of temperature and multiplicity.
A. Confluent BSC-40 cells were infected at moi = 5 with either wtVV (first two columns) or Cts9 (last two columns) at 31°C (top row) or 40°C (lower row). At 16h p.i., the monolayers were treated for 2 min with PBS at pH 7.4 or pH 4.6, as indicated above the panels, and incubated for a further 3h in complete medium before phase contrast photomicrography.
B. Effect of multiplicity on fusion mediated by wtVV or Cts9 at 31°C. BSC-40 cells were infected with wtVV (upper row) or Cts9 (lower row) at a multiplicity of 50 (left column), 5 (center column), or 0.5 (right column), incubated for 16h at 31°C, briefly acid-treated at pH 4.6, and photographed after 3h incubation in medium.
The amount of cell-cell fusion produced by acid-treated Cts9-infected cells at 31°C seemed reduced relative to similarly treated wtVV-infected cells (Fig. 6A). The extent of cell-cell fusion was compared visually at 31°C for acid-treated cells that had been infected with wtVV or Cts9 at multiplicities of 50, 5, and 0.5 (Fig. 6B). Fusion of wtVV-infected cells was similar and essentially complete at each of these multiplicities (Fig. 6B). In contrast, fusion mediated by Cts9 at multiplicities of 50 and 5 was extensive but still somewhat reduced compared with wtVV, and at an moi of 0.5, Cts9 produced very little fusion. These results suggest that Cts9 virions on the surface of infected cells are completely defective in mediating fusion from within at 40°C, and even at 31°C are partially defective.
Purified virions of Cts9 synthesized at 40°C cannot cause fusion from without
The ability of purified MV of Cts9 to cause fusion from without was tested by adsorbing virions directly to cells at a very high multiplicity or particle/cell ratio, subjecting the cells to low pH, and then incubating at 31°C to allow cell-cell fusion to occur. Cycloheximide was present throughout to minimize cytopathic effect. Actin was stained with fluorescent phalloidinAlexaFluor 568 to visualize the cytoskeleton, and nuclei were counterstained with DAPI. Acid-treated cells to which virus had not been added gave no fusion (Fig. 7A). Cells with adsorbed wt virus gave no fusion after treatment at pH 7.4 (Fig. 7B), but extensive fusion after low pH treatment, regardless of whether the virus had been produced at 31°C (Fig. 7C) or 40°C (Fig. 7D). Treatment of cells to which Cts9 virus had been adsorbed with pH 4.6 buffer resulted in a low level of fusion, with a few multinucleate cells evident (Fig. 7E) but none of the giant syncytia that were produced by wtVV (Fig. 7C). Adsorption of Cts9 MV grown at 40°C to cells gave no detectable fusion following acid treatment (Fig. 7F), indicating that these mutant virions were defective in fusion from without. The phenotype of the Cts9 mutant is therefore consistent with the A28 protein playing an essential role in the entry of mature virions into cells by a process involving fusion between the virus and cell membranes.
Figure 7. Fusion from without.

BSC-40 monolayers were mock-infected (A), or infected at moi = 300 (or with an equivalent number of non-infectious particles) in the presence of cycloheximide with purified MV of wtVV grown at 31°C (B, C) or with wtVV grown at 40°C (D), or with Cts9 produced at 31°C (E) or 40°C (F). Following virus adsorption and removal, all monolayers were briefly treated at pH 4.6 except for panel B, where monolayers were treated at pH 7.4. The cells were stained at 3h post-treatment with DAPI for nuclei (blue) and with phalloidin-AlexaFluor 568 for actin (red).
DISCUSSION
The experiments described here show that deletion of the C-terminal 14 amino acids of the vaccinia virus A28 protein results in a temperature sensitive phenotype. Cts9 virions produced at the non-permissive temperature are defective in virus fusion with the cell membrane and entry into cells. The Cts9 mutant in A28 was normal in terms of virus DNA and protein synthesis at 40°C, and no defects were observed in processing of the core proteins p4a (A10) or p4b (A3), or in virion morphogenesis (data not shown). However, mutant infections done at 40°C were defective in fusion from within, and mutant virions purified from non-permissive infections bound to cells but were defective for fusion from without and defective for virus entry as measured by the formation of intracellular cores. This phenotype is virtually identical to the phenotype described for a conditional lethal vaccinia mutant which, under non-permissive conditions, does not express A28 (Senkevich et al., 2004b); the same phenotype has been described for several other proteins associated with the entry/fusion complex (Senkevich & Moss, 2005; Townsley et al., 2005a; Townsley et al., 2005b; Izmailyan et al., 2006; Ojeda et al., 2006a; Ojeda et al., 2006b). The principal difference between the ts mutant described here and the inducible mutants described previously is that under non-permissive conditions, Cts9 produced virions which contain mutant A28 protein, whereas in the inducible mutants the A28 protein is absent from mutant virions. The simplest mechanistic interpretation of the Cts9 mutant phenotype is that the C-terminus of the A28 protein, including the C-terminal conserved cysteine, is important for assembly of a functional entry/fusion complex. Specifically we suggest that the C-terminus of A28 may contribute to the stability of the complex during assembly such that the truncated protein functions relatively normally in assembly at 31°C, but cannot assemble stably into the entry/fusion complex during morphogenesis at 40°C.
The finding that the Cts9 mutation truncated the A28 protein at the C-terminus but was not lethal at 31°C was unexpected in view of the strong conservation of the A28 protein (Senkevich et al., 2004a). It is noteworthy, however, that peptide tags can be fused to the A28 carboxy terminus without loss of function. Addition of the influenza hemagglutinin (HA) tag with serine (YPYDVPDYAS) to the C-terminus of A28 was tolerated (Senkevich et al., 2004a), and we have shown that a short linker plus the FLAG tag (LEDYKDDDDK) can similarly be appended to A28 to produce a viable virus (data not shown). The removal of the fourth conserved cysteine in Cts9 must disrupt one of the two conserved disulfide bridges in the A28 protein, and loss of this disulfide bridge appears to be detrimental to the functioning of the A28 protein at 40°C. It may be of interest in the future to assess the individual contributions of the four conserved cysteines to the structure and function of the entry/fusion complex.
The precise functioning of the A28 protein in entry remains to be determined. Although Cts9 virions made at 40°C bound normally to cells, binding may initially be mediated via a low-affinity attachment factor rather than directly to the putative cellular receptor which allows entry, and it is not clear if A28 (or any of the other proteins in the entry/fusion complex) interacts directly with the receptor. Future work is aimed at elucidating how A28 functions to mediate entry, a goal that may require identification of the cellular receptor to be fully realized.
MATERIALS AND METHODS
Cells and viruses
BSC40 cells (ATCC CRL-2761, epithelial cells derived from African green monkey kidney) were used for propagating and titering wild-type vaccinia virus strain WR and mutants Cts6 and Cts9 (Condit & Motyczka, 1981). CV-1 cells (ATCC CCL-70, African green monkey kidney cells) were used in experiments involving immunofluorescence microscopy. Cells infected with the ts mutants were grown at either 31°C (permissive temperature) or 40°C (nonpermissive temperature).
Marker rescue
Marker rescue was performed as previously described (Thompson & Condit, 1986; Meis & Condit, 1991). Briefly, cells were infected with mutant virus at an moi of approximately 0.03 pfu/cell, transfected with PCR products amplified from wt vaccinia DNA, incubated at 40°C for 4 days, and then stained with crystal violet. PCR products used in the rescue were generated using primers that precisely amplified individual open reading frames (ORFs) or clusters of adjacent ORFs. Integrated DNA Technologies, Inc. (IDT Coralville, IA) synthesized all oligonucleotides used as primers.
DNA sequence analysis
DNA sequence of the A27L and A28L genes from wt, Cts6, and Cts9 viruses was obtained by direct sequencing of PCR products amplified from total infected cell DNA. Qiagen DNeasy miniprep spin columns were used to isolate total infected cell DNA according to the manufacturer's instructions for isolation from cells in culture as previously described (Latner et al., 2000). DNA sequencing was done by the University of Florida ICBR DNA Sequencing Core Laboratory.
Purification of virus particles and measurement of particle/pfu ratio
Confluent monolayers of BCS40 cells in 150 mm dishes were infected with wt, Cts6, or Cts9 viruses at an moi of 10 and incubated at 31°C or 40°C. Infected cells incubated at 40°C were harvested at 24 h after infection while infected cells incubated at 31°C were harvested at 72 h after infection. Virus purification was performed by sedimentation through preformed sucrose density gradients as described (Ausubel et al., 1994). Purified virions were quantified by optical density at 260 nm (1 OD260 = 64 μg/ml virus = 1.2 × 1010 virus particles/ml). Infectivity of the purified virus was assayed by plaque titration on BSC40 cells at 31°C or 40°C.
Antibodies and immunoblotting
Rabbit polyclonal antiserum against the peptide DRRVQDVNDTISDVKQKWRC spanning amino acids 56-75 of A28 protein was generated by SigmaGenosys. Mouse mAb 5AG8 against A27 was obtained from David Ulaeto (DSTL Porton Down, UK), and rabbit antibodies against L1 and F17 from Paula Traktman (Medical College of Wisconsin, Milwaukee).
Purified virions (0.1 OD) were incubated at 37°C for 30 minutes in buffer containing 50 mM Tris and 1% NP40, with or without 50 mM DTT. Cores and membranes were isolated by centrifuging the virions in a microfuge for 30 minutes at 13,000 rpm and separating the supernatant from the pellet. Proteins were run on a 10-20% Tris-Tricine gel (BioRad) and visualized by immunoblotting with antibodies against A28, A27, L1, or F17.
Assessment of cytopathic effect
Infected cells were fixed with PBS containing 4% paraformaldehyde (PFA) for 10 minutes, permeabilized with PBS/0.05% Saponin, and stained with a 1:20 dilution of phalloidinAlexaFluor 568 (Molecular Probes, now Invitrogen) in PBS/1% BSA for 20 minutes. Texas Red filters were used to visualize stained cells.
Virus binding and entry
To assess virus binding, purified MV was added to CV-1 cells grown to 50% confluence in 8-chamber culture slides (BD Biosciences) at a multiplicity of 50, or an equivalent number of particles per cell, and adsorbed for 1h at 4°C. After washing 3 times to remove unbound virus, the cells were fixed with PBS/4% PFA for 10 min on ice, then 20 min at room temperature (RT). Following one wash with PBS, any remaining fixative was quenched with PBS/20 mM glycine for 5 min. The cells were blocked with PBS/10% fetal bovine serum (FBS) for 10 min at RT, and anti-A27 mAb 5AG8 added in PBS/10% FBS for 1h at RT. After 3 washes, goat anti-mouse FITC secondary antibody (Jackson ImmunoResearch Laboratories) diluted 1:100 in PBS/10% FBS was added for 30 min at RT. Following two PBS washes, cells were stained with PBS/0.05% Saponin containing 0.25 μg/ml DAPI, and bound virions visualized by fluorescence using a Zeiss Axiovert 200M microscope.
Entry of purified MV into cells was monitored by staining for intracellular cores with rabbit antibodies against the A4 core protein (from Mariano Esteban at the Centro Nacional de Biotecnología, Campus Universidad Autónoma, Madrid, Spain). CV-1 cells were infected as above at 4°C. After removing unadsorbed virus, medium containing 100 μg/ml cycloheximide was added, and the cells incubated at 31°C for 2h to allow entry to occur, or at 4°C as a negative control. Cells were fixed, quenched, and blocked as above, then permeabilized with PBS/0.1% Saponin for 10 min at RT. Rabbit anti-A4 serum diluted 1:250 in PBS/10% FBS was added for 1h at RT, and after washing goat anti-rabbit FITC (Jackson ImmunoResearch Laboratories) added. Nuclei were counterstained with propidium iodide, and imaged by fluorescence microscopy.
Fusion from within and without
Confluent monolayers of BSC-40 cells were infected with either wtVV or Cts9 at 31°C or 40°C, and at 18h p.i. washed with PBS, and treated for 2 min with PBS at either pH 7.4 or pH 4.6. After incubation for a further 3h in medium, the extent of fusion from within was assessed visually by phase contrast microscopy.
For fusion from without, purified MV was added to BSC-40 cells at moi = 300 (or an equivalent number of virus particles for Cts9 grown at 40°C), and allowed to adsorb for 1h at 4°C. After one PBS wash, cells were treated for 2 min at RT with warmed PBS at pH 7.4 or pH 4.6, then incubated for 4h at 31°C in medium containing 100 μg/ml cycloheximide. Cells were fixed with PBS/4% PFA, and stained with DAPI and phalloidin-AlexaFluor 568 in PBS/0.05% Saponin, and photographed by fluorescence microscopy.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Ulaeto (DSTL Porton Down, UK) for providing anti-A27 mAb 5AG8, Mariano Esteban (Centro Nacional de Biotecnología, Campus Universidad Autónoma, Madrid, Spain) for rabbit antibody against A4 protein, and Paula Traktman (Medical College of Wisconsin, Milwaukee) for rabbit antibodies against the L1 and F17 proteins. Support was provided to PCT and RWM from the Southeast Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley and Sons, Inc.; New York: 1994. [Google Scholar]
- Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J Virol. 2006;80:9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GC, Law M, Hollinshead M, Smith GL. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J Gen.Virol. 2005;86:1279–1290. doi: 10.1099/vir.0.80831-0. [DOI] [PubMed] [Google Scholar]
- Chung CS, Hsiao JC, Chang YS, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit RC, Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit RC, Moussatche N, Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv.Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–66. doi: 10.1016/0042-6822(90)90294-2. 517. [DOI] [PubMed] [Google Scholar]
- Gong S, Lai CF, Esteban M. Vaccinia Virus Induces Cell-Fusion at Acid Ph and This Activity Is Mediated by the N-Terminus of the 14-Kda Virus Envelope Protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmailyan RA, Huang CY, Mohammad S, Isaacs SN, Chang W. The Envelope G3L Protein Is Essential for Entry of Vaccinia Virus into Host Cells. J Virol. 2006;80:8402–8410. doi: 10.1128/JVI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SY, Bauer WR. Biosynthesis and phosphorylation of vaccinia virus structural protein VP11. Virology. 1987;159:399–407. doi: 10.1016/0042-6822(87)90479-x. [DOI] [PubMed] [Google Scholar]
- Latner DR, Xiang Y, Lewis JI, Condit J, Condit RC. The vaccinia virus bifunctional gene J3 (nucleoside-2′-O-)-methyltransferase and poly(A) polymerase stimulatory factor is implicated as a positive transcription elongation factor by two genetic approaches. Virology. 2000;269:345–355. doi: 10.1006/viro.2000.0243. [DOI] [PubMed] [Google Scholar]
- Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc.Natl.Acad.Sci.U.S.A. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74:3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttge BG, Moyer RW. Suppressors of a host range mutation in the rabbitpox virus serpin SPI-1 map to proteins essential for viral DNA replication. J Virol. 2005;79:9168–9179. doi: 10.1128/JVI.79.14.9168-9179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis RJ, Condit RC. Genetic and molecular biological characterization of a vaccinia virus gene which renders the virus dependent on isatin-beta-thiosemicarbazone (IBT) Virology. 1991;182:442–454. doi: 10.1016/0042-6822(91)90585-y. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Virology. Raven Press; New York: 2001. pp. 2849–2883. [Google Scholar]
- Ojeda S, Domi A, Moss B. Vaccinia virus G9 protein is an essential component of the poxvirus entry-fusion complex. J Virol. 2006a;80:9822–9830. doi: 10.1128/JVI.00987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S, Senkevich TG, Moss B. Entry of Vaccinia Virus and Cell-Cell Fusion Require a Highly Conserved Cysteine-Rich Membrane Protein Encoded by the A16L Gene. J Virol. 2006b;80:51–61. doi: 10.1128/JVI.80.1.51-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Rodriguez JR, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JF, Janeczko R, Esteban M. Isolation and Characterization of Neutralizing Monoclonal-Antibodies to Vaccinia Virus. Journal of Virology. 1985;56:482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ojeda S, Townsley A, Nelson GE, Moss B. Poxvirus multiprotein entry-fusion complex. Proc.Natl.Acad.Sci.U.S.A. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ward BM, Moss B. Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway. Journal of Virology. 2004a;78:2348–2356. doi: 10.1128/JVI.78.5.2348-2356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Ward BM, Moss B. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. Journal of Virology. 2004b;78:2357–2366. doi: 10.1128/JVI.78.5.2357-2366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, White CL, Koonin EV, Moss B. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc.Natl.Acad.Sci.U.S.A. 2002;99:6667–6672. doi: 10.1073/pnas.062163799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen.Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Condit RC. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986;150:10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Townsley AC, Senkevich TG, Moss B. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J Virol. 2005a;79:10988–10998. doi: 10.1128/JVI.79.17.10988-10998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley AC, Senkevich TG, Moss B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J Virol. 2005b;79:9458–9469. doi: 10.1128/JVI.79.15.9458-9469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley AC, Weisberg AS, Wagenaar TR, Moss B. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplasschen A, Hollinshead M, Smith GL. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J Gen.Virol. 1998;79(Pt 4):877–887. doi: 10.1099/0022-1317-79-4-877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.