Abstract
This study was designed to understand the basis for the efficacy of methylselenocysteine (MSC) in increasing the therapeutic index of irinotecan against human tumor xenografts. Nude mice bearing human FaDu and A253 tumors were treated orally with different doses of MSC and irinotecan. Plasma, tumor and normal tissue samples were collected at different times after MSC treatments and were analyzed for selenium (Se) concentration using electrothermal atomic absorption spectrophotometry. MSC is highly effective in modulating the therapeutic index of irinotecan in the treatment of human squamous cells carcinoma of the head and neck xenografts (FaDu and A253). Enhanced irinotecan efficacy was greater in FaDu tumors (100% CR) than in A253 tumors (60% CR), and depended on MSC dose with a minimum effective dose of 0.01 mg/d x 28. The highest plasma Se concentration was achieved 1 h after a single dose and 28 d after daily treatments of MSC. The ability of FaDu tumors to retain Se was significantly better than A253 tumors, and the highest Se concentration in normal tissue was achieved in the liver. Peak plasma and tissue Se concentrations were functions of the dose and duration of MSC treatment. The MSC-dependent increase in Se level in normal tissues may contribute to the protective effect against irinotecan toxicity observed in those tissues. Intratumoral total Se concentration was not found to be predictive of the combination therapy response rates. There is a critical need to develop a method to measure the active metabolite of MSC, rather than total Se.
Keywords: Methylselenocysteine, selenium, FaDu, A253, irinotecan
1. Introduction
Selenium (Se) is an essential trace element in human and animal nutrition. A significant body of animal model and epidemiological work indicates that Se also can reduce cancer risk [1–3]. Several mechanisms have been proposed for this anti-carcinogenic action: regulation of p53 by the Ref1 dependent redox mechanism [4, 5], induction of apoptosis associated with increased phosphorylation of p53 MAPK and dephosphorylation of Akt and ERK-1 and ERK-2 [6–8], and antiangiogenic activity by inhibiting the expression of vascular endothelial growth factors (VEGFs) [9], among others. Although most preclinical chemoprevention studies have used inorganic sodium selenite, the most informative human trial [10] used a Se-enriched yeast. Species of Se found in yeasts are reported to be dependent on the manufacturers and methods of detection. Although yeast Se is thought to be predominantly in a form of selenomethionine, other forms of yeast Se have been reported, such as selenocysteine, methylselenocysteine and unidentified selenium species [2, 11–13]. In the selenium intervention trial by Clark et al., administration of 200 μg of yeast Se was shown to reduce the incidence of several types of cancers [10, 14]. These results are consistent with most epidemiological studies showing Se status to be inversely associated with cancer risk [1, 15, 16]. Based on these data, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) was initiated to determine the effects of Se and vitamin E in preventing prostate cancer [17]. L-selenomethionine is one of the two intervention agents used in this trial.
Ip [2] has proposed that the anticarcinogenic actions of Se involve the formation of methylselenol (CH3SeH) as the active metabolite. Methylselenocysteine (MSC), a stable, water-soluble Se-compound that is quantitatively absorbed when taken orally [18], is then hydrolyzed by a β-lyase to yield methylselenol [2, 10, 19].
Previously, Azrak et al. [20] characterized untreated controls of A253 and FaDu human xenografts of the head and neck squamous cell carcinomas (HNSCC) using immunohistochemistry. A253 tumors are well-differentiated with an average doubling time of ~ 3.25 days. FaDu tumors are poorly-differentiated with an average doubling time of ~ 2.9 days. Cao et al. [21] reported that oral administration of MSC at the maximum tolerated dose (MTD, 0.2 mg/mouse) on a daily x 28 schedule (0.2 mg/d x 28), in combination with the weekly i.v. schedule of irinotecan (C33H38N4O6•HCl•3H2O) (100 mg/kg/wk x 4, MTD), increased the cure rate from 30% (irinotecan alone) to 100% in FaDu and from 10% to 60% in A253 xenografts. Oral pretreatment with MSC daily for seven days prior to initiation of irinotecan treatment was essential for optimal therapeutic synergy.
In this study, we determined that the enhanced antitumor activities of irinotecan and protections from its induced toxicity are MSC dose dependent. The minimum MSC dose that is needed to achieve 100% cure rates of irinotecan in FaDu xenografts was identified. In addition, we evaluated the pharmacokinetic profiles of Se in the plasma and select tissues after treatment with various doses of MSC, demonstrated that FaDu xenografts are capable of retaining Se more effectively than A253 xenografts, and determined whether Se levels are predictive of the observed therapeutic synergy of MSC and irinotecan.
2. Materials and Methods
2.1. Mice
Eight to 12-week-old, female athymic nude mice (nu/nu, body weight 20–25 g) were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). The mice were housed, five mice/cage, under specific pathogen-free conditions with water and food provided ad libitum, according to a protocol approved by the Institute Animal Care and Use Committee at Roswell Park Cancer Institute.
2.2. Tumors
Two human head and neck tumor xenografts (FaDu and A253) were used. Xenografts were initially established by implanting subcutaneously (s.c.) 106 cultured cells and passed several generations by transplanting ~ 50 mg non-necrotic tumor tissue before treatment, which began about one week after implantation, when the tumors were 100–200 mg in size (established tumors).
2.3. Drugs and treatments
Irinotecan was purchased from Pharmacia (Kalamazoo, MI), as a ready-to-use clinical formulation solution in 5 ml vials containing 100 mg of drug (20 mg/ml). Irinotecan was administered (100 and 200 mg/kg weekly x 4) by intravenously (i.v.) injection via the tail vein of animals. Methylselenocysteine hydrochloride (C4H9NO2Se·HCl) was purchased from Sigma (St. Louis, MO), as a powder in 100 mg/vial and dissolved in 0.9% NaCl at a final concentration of 1 mg/ml. It was administered daily by oral gavages (7 d prior to irinotecan administration and continued for 28 d) at different doses: 0.001, 0.005, 0.01, 0.05, 0.1, 0.2 (MTD) mg/d.
Treatments were administered to 10 mice in each group per experiment and every experiment was repeated twice. Cure rates (CR) were defined as no detectable tumor at the site of transplant for up to three months after termination of treatment. Plasma samples were collected: (a) 1 h, 2 h, 4 h, 8 h, 12 h, 16 h, and 24 h after treatment with single dose of MSC at 0.2 mg/d; (b) 2 h after treatment with MSC (0.2 mg/d) for 7, 14, 21 and 28 d; (c) 2 h after 7 d treatment with different doses of MSC (0.005, 0.01, 0.05, 0.1, 0.2 mg/d x 7). Tissue samples were collected at 2 h after 7 d treatment with various doses of MSC (0.005, 0.01, 0.05, and 0.2 mg/d x 7).
2.4. Total selenium measurement analysis
Total Se in plasma and tissues was measured by electrothermal atomic absorption spectrophotometry, using a PC-based ZL4100 Atomic Absorption Spectrophotometer (Perkin-Elmer) equipped with autosampler or automated Zeeman-effect background correction (Spectra AA-600, Varian Instruments, Walnut Creek, CA). Tissues were homogenized in 0.25% triton X-100 (10 ml diluent/1g tissue weight) using a Polytron tissue homogenizer (Brinkmann Instruments, Westbury, NY). Standard curves ranging from 40–800 ng/ml were prepared using the selenium atomic absorption spectroscopy analytical standard (Perkin-Elmer) or L-selenomethionine, and calibrated against standardized human plasma (Seronorm, Billingstad, Norway). Plasma and tissue homogenates were diluted 1:5 in a diluent consisting of 0.2% nitric acid, 0.1% Triton™ X-100, 1% Pd(NO3)2 and 0.1% Mg(NO3)2 prior to a 20 μl injection. The matrix modifiers Pd(NO3)2 and Mg(NO3)2 decreased the volatility of Se and prevented its loss during thermal pretreatment. They also increased the volatility of matrix components and promoted their removal before atomization. Matrix matched analytical standards were prepared identically in plasma and/or corresponding tissues with the diluent consisting of the matrix modifiers. The graphite furnace program consists of a two-step drying at 110°C and 130°C, pyrolysis at 1300°C, atomization at 2100°C and clean out at 2400°C. Two concentrations (600 ng/ml and 100 ng/ml) from the standard curves prepared from tissue homogenate were processed in duplicate and used as quality control (QC) samples for tissue samples. Other QC samples, including plasma spiked with selenite or L-selenomethionine at two different concentrations (one at the high end of the standard curve (500 ng/ml) and one at the low end of the standard curve (150 ng/ml)) were run with the test plasma samples. The standard procedure called for repeated analysis of samples for which the corresponding QC sample showed a coefficient of variation (CV) greater than established acceptable values (i.e., >15% for high-QCs; by >20% for low-QCs).
2.5. Pharmacokinetics data analysis
Se pharmacokinetic parameters were estimated by standard non-compartmental methods (WinNonlin Version 5.0, Pharsight Corporation). Briefly, the terminal elimination rate constant (ke) was computed by least squares linear regression, and half-life was computed by 0.693/ke. Area under the concentration time curve extrapolated to infinity (AUC0-∞) was computed using the trapzoidal rule. For this analysis, data were pooled with the arithmetic mean Se value at each time point used to estimate pharmacokinetic parameters. The baseline (pre-dose) Se concentration, reflective of endogenous Se levels, was subtracted from each measured Se concentration. Thus, the resultant oral volume of distribution, oral clearance, and AUC represent the changes in plasma Se following MSC supplementation. Since the dose of MSC administered was for the hydrochloride salt, the dose used for the pharmacokinetic analysis was corrected to reflect the MSC base.
2.6. Statistical analysis
Data were analyzed using SAS (SAS Institute, Raleigh, NC) by the ANOVA test, and means were compared post-hoc using the New Multiple Range test. The results of the comparisons were considered statistically significant when the p-value was less than 0.05.
3. Results
3.1. Enhancement of irinotecan therapeutic index is MSC dose dependent
Daily treatment with irinotecan alone (200 mg/kg/wk x 4, double the MTD) resulted in 50% of nude mice lethality. Administration of daily MSC (0.01, 0.05, 0.1, or 0.2 mg/d x 28) starting 7 d prior to irinotecan resulted in complete protection from irinotecan-induced death in nude mice (100% survived the combination treatment). Treatment with lower doses of MSC (0.001 or 0.005 mg/d x 28) increased the nude mice survival rate to 60% and 80%, respectively (Fig. 1A).
Fig. 1. The minimum effective dose of MSC to prevent toxicity (1A) and augment anti-tumor activity of irinotecan (1B).
MSC is highly effective modulator of the therapeutic index of irinotecan in human squamous cells carcinoma of the head and neck xenografts and this efficacy is MSC dose dependent. In figure 1A, administration of weekly irinotecan (200 mg/kg x 4, double the MTD), resulted in 50% nude mice lethality. MSC dose of 0.01 mg/d x 28 is the minimum dose to achieve 100% protection of nude mice from irinotecan induced toxicities.
In Figure 1B, administration of weekly irinotecan (100 mg/kg x 4, MTD) resulted in 30% cure rate (CR) in FaDu tumors and 10% CR in A253. Administration of MSC alone resulted in 0% CR in both tumors but reduced tumor growth by ~ 30% (data not shown). The minimum MSC dose to achieve 100% CR in combination with irinotecan in FaDu tumors was 0.01 mg/d x 28, whereas MSC dose of 0.2 mg/d x 28 was needed to increase the cure rate of irinotecan to 60% in A253.
Treatment of FaDu tumors with the combination of MSC (0.005mg/d x 28) and irinotecan (100 mg/kg/wk x 4, MTD) increased the cure rate from 30% (irinotecan alone) to 80% (Fig. 1B). The maximum cure rate of combination treatment of 100% was achieved when MSC administered at 0.01, 0.05, 0.1, or 0.2 mg/d x 28 in combination with irinotecan (Fig. 1B). In A253 tumors, a higher dose of MSC (0.05 mg/d x 28) was needed to double the cure rate from 10% with irinotecan alone to 20% after the combination treatment. Escalating the dose of MSC to the MTD of 0.2mg/d x 28 resulted in only 60% cure rate after the combination treatment (Fig. 1B). MSC alone yielded no tumor cure rate (0% in both tumors), but reduced tumor growth by ~ 30% (data not shown).
3.2. Pharmacokinetics of plasma Se concentration after single oral dose of MSC
The average plasma Se concentration in untreated controls was 4.1±0.1 μmol/L. After treatment with MSC (0.2 mg/d x 1) and normalization to untreated controls, plasma Se concentration peaked 1 h after treatment reaching a maximum concentration (Cmax) of 8.9±0.2 μmol/L (P<0.001, compared to untreated control, Fig. 2A). Plasma Se concentration 24 h after MSC treatment (1.2±0.2 μmol/L) continued to be significantly higher than the controls (P<0.01, Fig. 2A). The area under the Se concentration time curve (AUC0-∞) was 79.95 μmol/L*h; Se half-life was 7.9 h with volume of distribution of 0.33 L and clearance rate of 0.48 ml/min (Table 1).
Fig. 2. Plasma selenium concentrations in mice at various times after treatment with MSC (0.2 mg/d).
In figure 2A, after single dose treatment of MSC at 0.2 mg/d and normalizing to untreated controls, plasma Se concentration peaked at 1 h (Cmax = 8.9±0.2 μmol/L) with a half life (t½) of 7.9 h.
In figure 2B and after normalizing to untreated controls, Se concentration reached the highest (11.5±0.2 μmol/L) at 2 h after 28 d treatment with MSC at 0.2 mg/d. Micro-Molar concentration (μM) was calculated by dividing plasma Se concentration in ng/ml over Se molecular weight of 79. * p <0.05, ** p <0.01, *** p<0.001 when compared to untreated control; † p <0.05, †† p <0.01, ††† p <0.001 when compared to day 1; ‡ p <0.05, ‡‡ p <0.01, ‡‡‡ p <0.001 when compared to day 7; ¥ p <0.05, ¥¥ p <0.01, ¥¥¥ p <0.001 when compared to day 21.
Table 1.
Plasma pharmacokinetic parameters of selenium after a single dose of oral MSC treatmenta
MSC dose (0.2 mg/d x 1)
Maximum observed Se concentration
Half-life
Area under the Se plasma concentration-time curve due to MSC supplementation
Apparent Oral Volume of distribution
Apparent Oral Clearance
3.3. Kinetics of plasma Se concentration
Nude mice were treated with MSC (0.2 mg/d) and plasma Se concentrations were measured at day 1, 7, 14, 21 and 28 of treatment. After normalization to Se concentration in untreated controls (4.2±0.1 μmol/L), the average plasma Se concentration significantly increased to: 5.9±0.5 μmol/L after 1 d MSC treatment; 6.1±0.3 μmol/L after 7 d MSC treatment; 8.1±1.4 μmol/L after 14 d MSC treatment; 8.5±0.6 μmol/L after 21 d MSC treatment, and 11.5±0.2 μmol/L after 28 d of MSC treatment (P<0.001, compared to untreated controls, Fig. 2B). The increase in the plasma Se concentration at 28 days was statistically significant (P<0.05), when compared with concentrations on days 1, 7, and 21 (Fig. 2B).
3.4. Plasma Se concentrations after seven days treatment with various doses of MSC
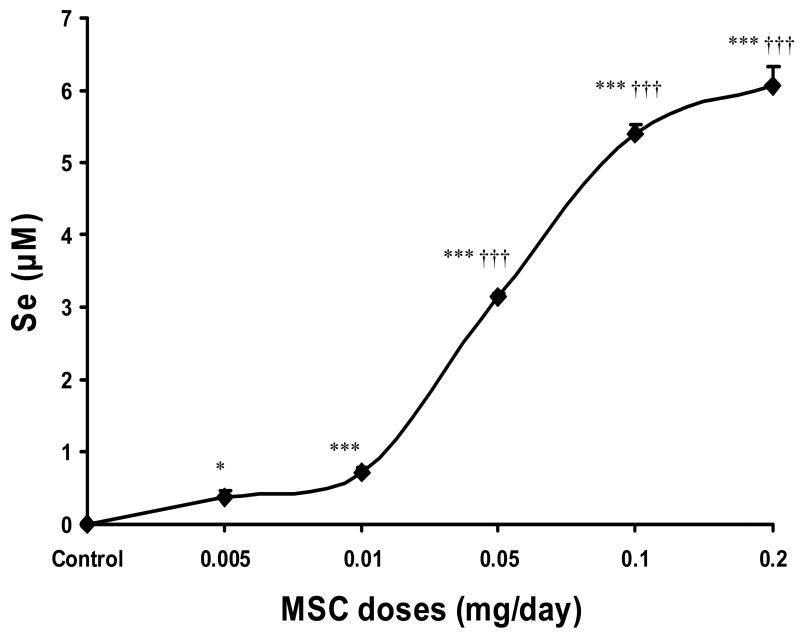
Nude mice were treated for 7 d with different doses of MSC and plasma Se concentrations were measured at 2 h after the last dose of MSC. After normalization to Se levels in untreated controls (4.2±0.1 μmol/L), treatment with MSC (0.01, 0.05, 0.1 or 0.2 mg/d x 7) significantly increased (P<0.001) plasma Se concentration, when compared with untreated controls (Fig. 3). This increase in plasma Se concentrations were also significant (P<0.001), when compared to 0.005 values (0.38±0.1 μmol/L) except for Se values after 0.01 MSC treatment (Fig. 3). The lowest pretreatment level of plasma Se associated with the complete response of the combination treatment in FaDu tumors was 0.7±0.1 μmol/L.
Fig. 3. Plasma selenium concentrations 2 h after 7 d treatment with various doses of MSC.
The required plasma Se concentration before starting irinotecan to achieve the optimal therapeutic index is ranged from 0.7±0.1 μmol/L after MSC 0.01 mg/d x 7 to 6.1±0.3 μmol/L after MSC 0.2 mg/d x 7. * p <0.05, ** p <0.01, *** p<0.001 when compared to untreated control; † p <0.05, †† p <0.01, ††† p <0.001 when compared to 0.005.
3.5. Total selenium concentration in FaDu and A253 tumors after seven days treatment with various doses of MSC
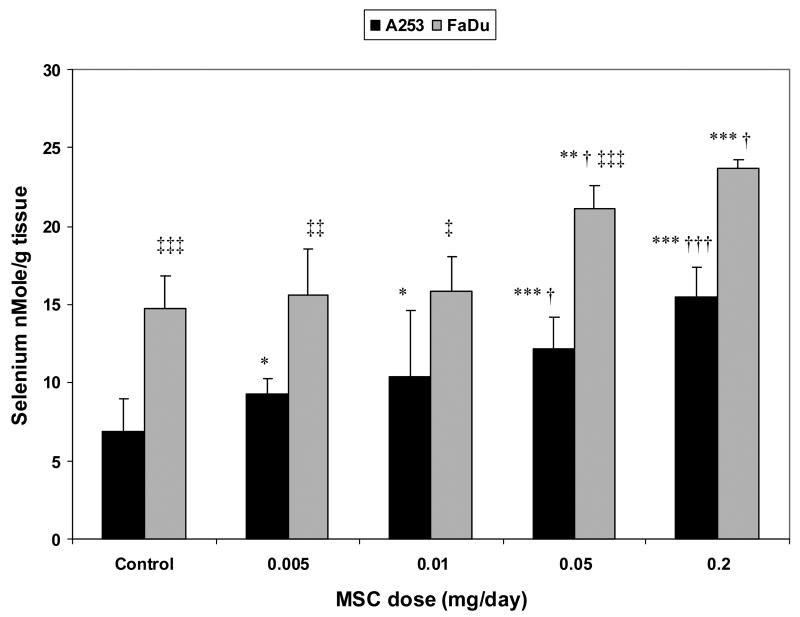
Nude mice bearing human xenografts of A253 and FaDu were treated with various doses of MSC for seven days and intratumoral Se concentrations were measured. The basal Se concentration in A253 tumors was 6.9±2.1 nmol/g. This value increased to 9.3±0.9, 10.4±4.2, 12.2±2, and 15.5±1.9 nmol/g after seven-day treatment with MSC at doses of 0.005, 0.01, 0.05 and 0.2 mg/d, respectively. The increases in Se concentrations in A253 tumors, except for the value after 0.01 mg/d x 7, were significant (P<0.05), when compared with the Se value in A253 tumors after the lowest MSC treatment (0.005 mg/d x7) (Fig. 4).
Fig. 4. Intratumoral selenium concentrations 2 h after 7 d treatment with various doses of MSC.
Intratumoral Se concentration is always higher in FaDu tumor (more responsive) than in A253 (less responsive) after treatments with various doses of MSC. The minimum FaDu Se concentration after MSC treatment, which is required to enhance irinotecan anti-tumor activity to 100%, is 15.8±2.2 nmol/g. A253 Se concentration was 10.4±4.2 nmol/g after same dose and treatment of MSC but increased to 15.5±1.9 nmol/g after treatment with MSC at 0.2 mg/d x 7. * p <0.05, ** p <0.01, *** p<0.001 when compared to untreated control; † p <0.05, †† p <0.01, ††† p <0.001 when compared to 0.005 in each tumor; ‡ p <0.05, ‡‡ p <0.01, ‡‡‡ p <0.001 when compared to A253.
The Se concentration of untreated FaDu tumors was 14.8±2.1 nmol/g. This value increased to 15.6±3.0, 15.8±2.2, 21.1±1.5, and 23.7±0.6 nmol/g after seven-day treatment with MSC at doses of 0.005, 0.01, 0.05, and 0.2 mg/d, respectively. These increases in Se concentration in FaDu tumors, except the value after 0.01 mg/d x 7, were significant (P<0.005), when compared to the 0.005 treatment values (Fig. 4).
3.6. Selenium concentrations in normal tissue after seven days treatment with various doses of MSC
Normal tissue from the above treated nude mice were collected and Se concentrations of normal tissue (liver, kidneys, small intestine, large intestine and bone marrow) after seven-day treatment with different doses of MSC (0.005, 0.01, 0.05 and 0.2 mg/d) were measured. After treatment with the highest of MSC dose (0.2 mg/d x 7), the liver had the highest Se concentration of 134.3±27.4 nmol/g, followed by kidneys (56.4±12.8 nmol/g), small intestine (29.2±4.3 nmol/g), bone marrow (13.2±0.8 nmol/g) and large intestine (11.8±1.2 nmol/g) (Table 2). With the exception of large intestine, these values were significantly different (P<0.005) from those obtained after treatment with the lowest dose of MSC administered (0.005 mg/d x 7) (Table 2).
Table 2.
Selenium concentrations in normal tissue after treatment with various doses of MSC
| Selenium level in1 | MSC treatment2 | ||||
|---|---|---|---|---|---|
| Control | 0.0053 | 0.01 | 0.05 | 0.2 | |
| Liver | 23.6±4.9*** | 36.5±3.9*** | 37.9±3.5*** | 92.2±12.8***††† | 134.3±27.4***††† |
| Kidneys | 22±5.4 | 29.7±1.5* | 30.3±2.5* | 37.2±6.4***† | 56.4±12.8***††† |
| Small intestine | 9.7±4.2 | 12.2±5.6 | 15.6±4.2* | 16.9±5.6* | 29.2±4.3***††† |
| Large intestine | 6.6±2.9 | 7.7±6 | 13.2±5.3* | 11.2±2.6* | 11.8±1.2** |
| Bone marrow | 7.5±1.9 | 8.3±3 | 8.2±4.8 | 8.4±1.3 | 13.2±0.8***† |
nmole/g tissue
9 mice per treated group
MSC dose (mg/d x 7)
P<0.05 when compared to untreated control
P<0.01 when compared to untreated control
P<0.001 when compared to untreated control
P<0.05 when compared to MSC treatment at 0.005
P<0.01 when compared to MSC treatment at 0.005
P<0.001 when compared to MSC treatment at 0.005
4. Discussion
Chemotherapy with irinotecan, a topoisomerase I poison, alone and in combination with other anticancer drugs is limited by lack of therapeutic selectivity and resistance. Saltz et al. [18] reported that the response rate with irinotecan/5-FU/leucovorin combination therapy for patients with advanced colorectal cancer was 39%, with a median survival of 15.9 months. Although the risk of tumor progression was reduced by 36% with irinotecan/5-FU/leucovorin combination therapy, irinotecan-induced gastrointestinal toxicities were more common [22]. The challenge is to develop new drugs and treatment modalities that will significantly impact cure rates, but with minimal toxicity. Cao et al. [21] demonstrated that the combination of MSC administered orally in a non-toxic dose (MTD) and schedule (7 d prior to weekly i.v. schedule of irinotecan) significantly increased the cure rates of human head and neck xenografts and protected animals from irinotecan-induced death.
In this study, we determined the minimum dose of MSC needed to completely protect mice from irinotecan-induced death to be 0.01 mg/d for 28 d (5% of MSC’s MTD). Further, a 50% reduction of this dose of MSC under this protocol still resulted in 80% protection from irinotecan-induced death (Fig. 1A). In FaDu tumors, enhanced irinotecan antitumor activity resulting in complete cure (100% CR) was achieved when MSC was administered at 0.01mg/d for 28 d, in combination with irinotecan administered weekly at 100 mg/kg/week for four weeks. In contrast, the same dose of MSC did not enhance cure rate in A253 tumors (Fig. 1B). In fact, the best cure rate for A253 tumors, 60% CR, was achieved when MSC was administered at the MTD (Fig. 1B). These results show that MSC is highly effective in modulating the therapeutic index of irinotecan in the treatment of human squamous cells carcinoma of the head and neck xenografts. Enhanced irinotecan efficacy was greater in FaDu than in A253 tumors, and depended on both MSC dose (0.01 mg/d/mouse) and schedule (requiring 7 d pretreatment) (Fig. 1).
Azrak et al. [23] previously evaluated and compared the expression of multiple molecular markers in untreated controls of A253 and FaDu xenografts that were known to be associated with the irinotecan metabolic pathway. Briefly, no significant difference (p<0.05) in expression levels of those investigated markers was associated with the enhanced therapeutic efficacy of irinotecan except decreased expressions of ATP binding cassette transporters (ABCC1 and ABCG2, efflux pumps of SN-38 the active metabolite of irinotecan) and SN-38 resistant gene developmentally regulated GTP binding protein 1 (DRG1). In addition, poorly-differentiated tumors with slower doubling time (FaDu) seemed to be more sensitive to the combination therapies involving irinotecan [20]. In attempt to understand the mechanism associated with the observed synergy, the plasma and intratumoral pharmacokinetic parameters representing changes in total Se due to MSC supplementation were measured. Direct measurement of MSC pharmacokinetic parameters was not possible because there is no available assay for MSC measurements.
Last et al. [24] demonstrated that the overall survival in non-Hodgkin lymphoma patients is correlated to the basal concentration of Se in their plasma, which could be considered as a prognostic marker and predictive of dose delivery. In this study, the peak of plasma Se level (Cmax) was achieved 1 h after a single dose of MSC (0.2 mg/d) with a relatively long half-life of 7.9 h (Table 1). Data presented in Fig. 2B demonstrated that plasma Se reached the highest level after daily treatment of MSC at the MTD for 28 days. The average concentration of Se in plasma after a full course of daily treatments of MSC (from day 1 to day 28) was 8.6±0.6 μmol/L. Seven-day pretreatment with MSC doses that completely protected animals from irinotecan induced death (from 0.01 to 0.2 mg/d, Fig. 1A) significantly increased (p<0.0005) plasma Se concentrations, ranging from 17% after 0.01 mg/d to 145% after 0.2 mg/d, when compared to untreated control (Fig. 3). These data indicate that the maximum increase in irinotecan therapeutic index corresponded with a minimum plasma Se concentration of 0.7±0.1 μmol/L. However, administration of a lower dose of MSC (0.005 mg/d, 80% protection, Fig. 1A) did not significantly increase plasma Se concentration, when compared with untreated controls or 0.01 mg/d of MSC (Fig. 3). This could be explained as a result of inter individual variability between mice; the fact that 20% of mice were not protected with this dose of MSC could act as cofounders contributing to the non-significant difference and skewing Se measurements.
Although reaching a plasma Se concentration before treatment with irinotecan might be used as a guide for protection from irinotecan-induced toxicity, Se concentration in normal tissue is the crucial determining factor for protection from toxicity. Administration of MSC at 0.01 mg/d significantly increased normal tissue Se concentrations (p<0.05), except for bone marrow, when compared to untreated controls (Table 2). These tissue Se concentrations continued to be significantly different, with higher doses of MSC, when 100% protection was achieved. However, administration of MSC (0.005 mg/d, 80% protection, Fig. 1A) did not significantly increase the Se concentration in normal tissue, when compared to untreated controls or 0.01 mg/d MSC, except for the liver and kidneys (Table 2). The minimum Se concentration required to protect normal tissues from irinotecan-induced death before irinotecan administration appears to be: 37.9±3.5 nmol/g in the liver; 30.3±12.5 nmol/g in the kidneys; 15.6±4.2 nmol/g in the small intestine; 13.2±5.3 nmol/g in the large intestine; and 8.2±4.8 nmol/g in the bone marrow (Table 2). The fold change of normal tissue Se concentrations after MSC (0.2 mg/d x 7) over the concentration in untreated controls varied from 1.8-fold for bone marrow to 5.7-fold for liver (Table 3). These results indicate that MSC treatment increased Se concentrations in both the plasma and in normal tissues.
Table 3.
Fold change of selenium level at day 7 in tissue after treatment with MSC (0.2 mg/d x 7) to untreated control.
| Samples | Fold change of 0.2/control |
|---|---|
| A253 | 2.2 ± 0.4 |
| FaDu | 1.6 ± 0.1 |
| Liver | 5.7 ± 2.3 |
| Kidneys | 2.6 ± 1.0 |
| Small Intestine | 3.0 ± 0.4 |
| Large Intestine | 1.8 ± 0.2 |
| Bone Marrow | 1.8 ± 0.2 |
Two other reports are relevant to the present findings. Beguin et al. [25, 26] reported that tumors could, depending on “tumor activity,” retain Se and serum Se level in patients with acute non-lymphocytic leukemia. Gianduzzo et al. [27] showed that men who received daily oral Se supplementation for 30 d have statistically significant higher concentrations of Se in the prostate than those receiving a placebo, although the Se levels in the prostate and peripheral blood were poorly correlated. In this study, 7 d treatment with escalating doses of MSC was associated with sequential increases of intratumoral Se concentrations in both FaDu and A253 tumors (Fig. 4). These data suggest that both FaDu and A253 tumors have capabilities of accumulating Se after administration of MSC. Further, in FaDu tumors, 7 d treatment with MSC (0.01 mg/d, 100% CR) corresponded with Se concentration of 15.8±2.2 nmol/g. However, in A253 tumors, using the same MSC dose and schedule resulted in a lower Se concentration (10.4±4.2 nmol/g) and did not modulate irinotecan anti-tumor activity (10% CR, Fig. 1). In fact, intratumoral Se concentrations were always significantly higher in untreated FaDu controls than in untreated A253 controls and after administration of the same dose of MSC to both tumors (Fig. 4). The fold change of intratumoral Se concentration of FaDu over A253 tumors ranged from 2.1-fold in untreated controls to a significant increase (P<0.05) of an average of 1.6-fold after every MSC dose used (Table 3). These data indicate that the capability of FaDu tumor to retain Se is significantly better (p<0.05) than A253 tumors. Although administration of MSC (0.01 mg/d) increased the therapeutic efficacy of irinotecan (Fig. 1B) in FaDu tumors, there was no significant increase in Se concentration in FaDu tumors after this dose when compared to Se concentration after MSC dose of 0.005 mg/d (Fig. 4). However, Se concentrations in FaDu tumors was significantly increased after higher doses of MSC (0.05 and 0.2 mg/d) when compared to Se concentration after the same dose of MSC (0.005 mg/d, Fig. 4). On the other hand, in A253 tumors, the cure rate increased only with these higher doses of MSC, which also increased Se levels in A253 tumors (Fig. 4). This could be attributed to the insensitivity of the only available method to measure Se. Thus, the present methodology of measuring total Se concentration is not sufficiently sensitive and precludes drawing a conclusion of association between intratumoral total Se concentration and response rate at lower doses of MSC. The development of a method to detect the active metabolite of Se, rather than detecting total Se concentration is greatly needed.
In summary, MSC, in a dose dependent fashion, is highly effective in modulating the therapeutic index of irinotecan in the treatment of FaDu than A253 tumors. Results suggest that achievement of critical plasma and normal tissue concentrations of Se may be necessary to achieve optimal protection of normal tissue form irinotecan-induced death. To that end, tissue and normal Se levels could be used to guide the high Se doses, but not the low doses, needed to be administered in order to evaluate potential for impact on the therapeutic selectivity of anticancer drugs. Based on these preclinical data, a phase I clinical trial of combination of MSC and irinotecan is under development. Finally, this study raises an important and interesting issue of whether different forms of Se (e.g., selenite or selenomethionine) would have different protective effects that cannot be explained on the basis of their different abilities to support tissue Se concentrations and also the need for a specific and sensitive method to measure the active metabolite of MSC (methylsenlol).
Acknowledgments
We thank Kevin A. Craig for his editorial assistance with this manuscript.
Supported in part by project grant CA65761 and an Institute Comprehensive Cancer Center
Support Grant CA16056 from the National Cancer Institute, Bethesda, MD
Abbreviations
- MSC
methylselenocysteine
- Se
selenium
- MTD
maximum tolerated dose
- CR
cure rates
- s.c
subcutaneously
- i.v.
intravenously
- QC
quality control
- AUC
area under the concentration time curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Bayoumy K. The role of selenium in cancer prevention. In: DeVita VT, Hellman S, Rosenberg SA, editors. Principles and Practice of Oncology. 4. Philadelphia, PA: Lippincott; 1994. pp. 1–15. [Google Scholar]
- 2.Ip C. Lessons from basic research in selenium and cancer prevention. Journal of Nutrition. 1998;128:1845–54. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 3.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacology & Therapeutics. 1998;79:179–92. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 4.Gudkov AV. Converting p53 from a killer into a healer. Nature Medicine. 2002;8:1196–8. doi: 10.1038/nm1102-1196. [DOI] [PubMed] [Google Scholar]
- 5.Seo Y, Kelley M, Smith M. Selenomethionine regulation of p53 by a Ref1-dependent redox mechanism. Proc Nat'l Acad Sci. 2002;99:14548–14553. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Ganther HE, Stewart C, Ip C. Identification of molecular targets associated with selenium-induced growth inhibition in human breast cells using cDNA microarrays. Cancer Research. 2002;62:708–14. [PubMed] [Google Scholar]
- 7.Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Research. 2003;63:52–9. [PubMed] [Google Scholar]
- 8.Ip C, Dong Y. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Research. 2001;21:863–7. [PubMed] [Google Scholar]
- 9.Lu J, Jiang C, Kaeck M, Ganther H, Vadhanavikit S, Ip C, et al. Dissociation of the genotoxic and growth inhibitory effects of selenium. Biochemical Pharmacology. 1995;50:213–9. doi: 10.1016/0006-2952(95)00119-k. [DOI] [PubMed] [Google Scholar]
- 10.Clark L, Combs GJ, Turnbull B, Slate E, Chalker D, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1997;276:1957–1963. Erratum in: JAMA 1997;277(19):1520. [PubMed] [Google Scholar]
- 11.Nelson MA, Porterfield BW, Jacobs ET, Clark LC. Selenium and prostate cancer prevention. Seminars in Urologic Oncology. 1999;17:91–6. [PubMed] [Google Scholar]
- 12.Lamson DW, Brignall MS. Natural agents in the prevention of cancer, part two: preclinical data and chemoprevention for common cancers. Alternative Medicine Review. 2001;6:167–87. [PubMed] [Google Scholar]
- 13.Larsen EH, Hansen M, Paulin H, Moesgaard S, Reid M, Rayman M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. Journal of AOAC International. 2004;87:225–32. [PubMed] [Google Scholar]
- 14.Lamson DW, Brignall MS. Natural agents in the prevention of cancer. Part 1: human chemoprevention trials. Alternative Medicine Review. 2001;6:7–19. [PubMed] [Google Scholar]
- 15.Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, et al. Prediagnostic serum selenium and risk of cancer. Lancet. 1983;2:130–4. doi: 10.1016/s0140-6736(83)90116-2. [DOI] [PubMed] [Google Scholar]
- 16.Alaejos MS, Diaz Romero FJ, Diaz Romero C. Selenium and cancer: some nutritional aspects. Nutrition. 2000;16:376–83. doi: 10.1016/s0899-9007(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 17.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. Journal of Urology. 2001;166:1311–5. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 18.Ip C, Zhu Z, Thompson HJ, Lisk D, Ganther HE. Chemoprevention of mammary cancer with Se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Research. 1999;19:2875–80. [PubMed] [Google Scholar]
- 19.Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Molecular Carcinogenesis. 2002;34:113–20. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- 20.Azrak RG, Cao S, Slocum HK, Toth K, Durrani FA, Yin MB, et al. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clinical Cancer Research. 2004;10:1121–9. doi: 10.1158/1078-0432.ccr-0913-3. [DOI] [PubMed] [Google Scholar]
- 21.Cao S, Durrani F, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clinical Cancer Resreach. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 22.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. New England Journal of Medicine. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [see comment] [DOI] [PubMed] [Google Scholar]
- 23.Azrak RG, Yu J, Pendyala L, Smith PF, Cao S, Li X, et al. Irinotecan pharmacokinetic and pharmacogenomic alterations induced by methylselenocysteine in human head and neck xenograft tumors. Molecular Cancer Therapeutics. 2005;4:843–54. doi: 10.1158/1535-7163.MCT-04-0315. [DOI] [PubMed] [Google Scholar]
- 24.Last KW, Cornelius V, Delves T, Sieniawska C, Fitzgibbon J, Norton A, et al. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2003;21:2335–41. doi: 10.1200/JCO.2003.06.145. [see comment] [DOI] [PubMed] [Google Scholar]
- 25.Beguin Y, Weber G. Serum selenium in lymphoma. Journal of Clinical Oncology. 2004;22:3429. doi: 10.1200/JCO.2004.99.240. [comment] author reply 3430. [DOI] [PubMed] [Google Scholar]
- 26.Beguin Y, Bours V, Delbrouck JM, Robaye G, Roelandts I, Bury J, et al. Relationship of serum selenium levels to tumor activity in acute non-lymphocytic leukemia. Carcinogenesis. 1989;10:2089–91. doi: 10.1093/carcin/10.11.2089. [DOI] [PubMed] [Google Scholar]
- 27.Gianduzzo TR, Holmes EG, Tinggi U, Shahin M, Mactaggart P, Nicol D. Prostatic and peripheral blood selenium levels after oral supplementation. Journal of Urology. 2003;170:870–3. doi: 10.1097/01.ju.0000081052.51707.cf. [DOI] [PubMed] [Google Scholar]