Abstract
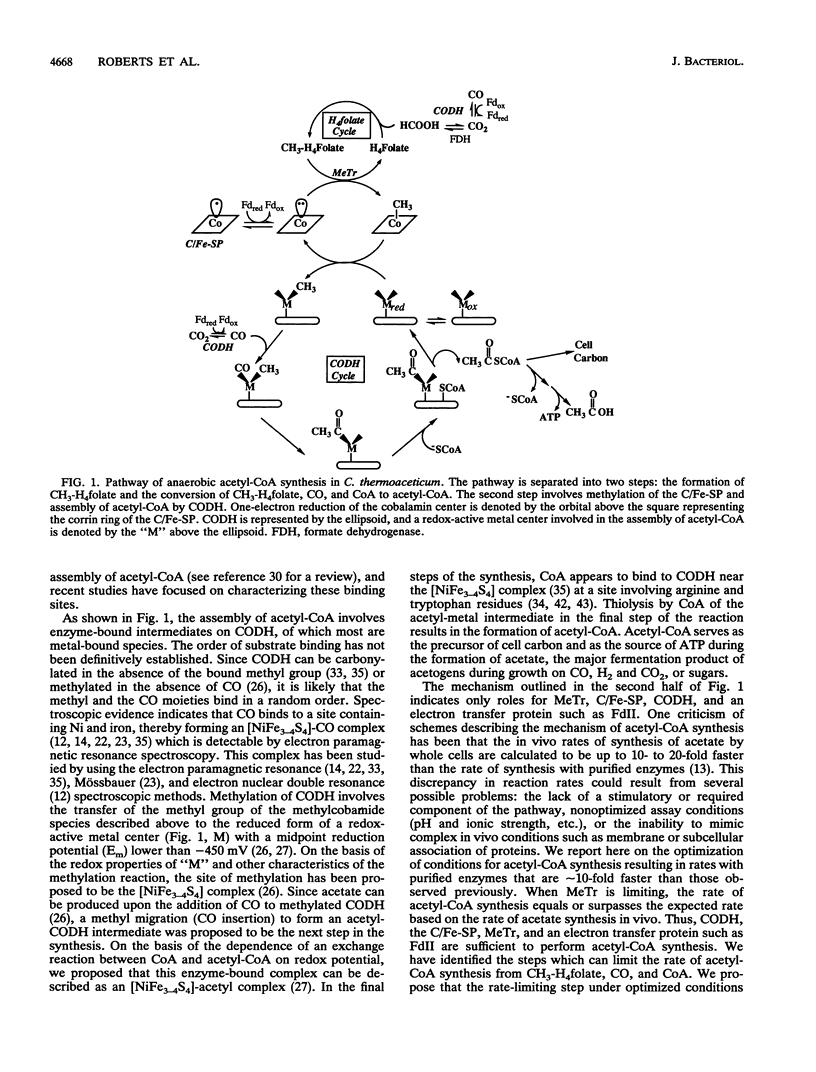
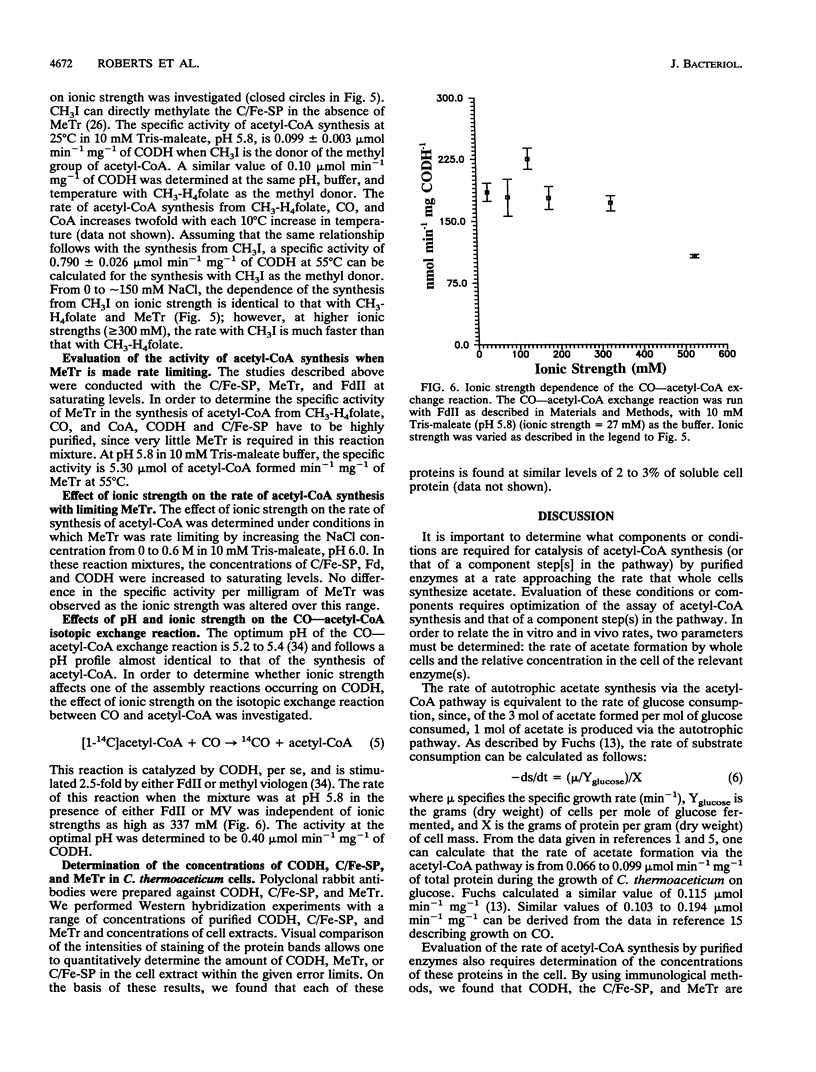
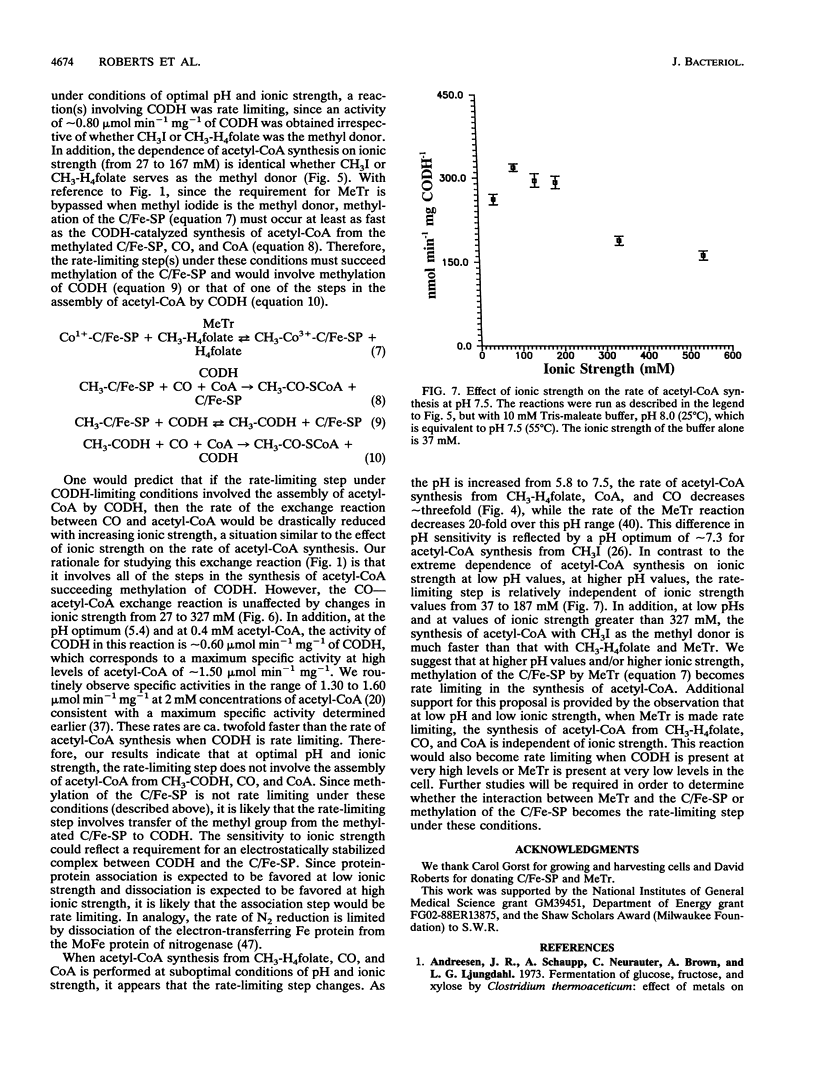
Many anaerobic bacteria fix CO2 via the acetyl-coenzyme A (CoA) (Wood) pathway. Carbon monoxide dehydrogenase (CODH), a corrinoid/iron-sulfur protein (C/Fe-SP), methyltransferase (MeTr), and an electron transfer protein such as ferredoxin II play pivotal roles in the conversion of methyltetrahydrofolate (CH3-H4folate), CO, and CoA to acetyl-CoA. In the study reported here, our goals were (i) to optimize the method for determining the activity of the synthesis of acetyl-CoA, (ii) to evaluate how closely the rate of synthesis of acetyl-CoA by purified enzymes approaches the rate at which whole cells synthesize acetate, and (iii) to determine which steps limit the rate of acetyl-CoA synthesis. In this study, CODH, MeTr, C/Fe-SP, and ferredoxin were purified from Clostridium thermoaceticum to apparent homogeneity. We optimized conditions for studying the synthesis of acetyl-CoA and found that when the reaction is dependent upon MeTr, the rate is 5.3 mumol min-1 mg-1 of MeTr. This rate is approximately 10-fold higher than that reported previously and is as fast as that predicted on the basis of the rate of in vivo acetate synthesis. When the reaction is dependent upon CODH, the rate of acetyl-CoA synthesis is approximately 0.82 mumol min-1 mg-1, approximately 10-fold higher than that observed previously; however, it is still lower than the rate of in vivo acetate synthesis. It appears that at least two steps in the overall synthesis of acetyl-CoA from CH3-H4folate, CO, and CoA can be partially rate limiting. At optimal conditions of low pH (approximately 5.8) and low ionic strength, the rate-limiting step involves methylation of CODH by the methylated C/Fe-SP. At higher pH values and/or higher ionic strength, transfer of the methyl group of CH3-H4folate to the C/Fe-SP becomes rate limiting.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. R., Graves M. C., Feinberg B. A., Ragsdale S. W. Characterization of the recombinant Clostridium pasteurianum ferredoxin and comparison of its properties with those of the native protein. Biofactors. 1990 Jul;2(3):197–203. [PubMed] [Google Scholar]
- Daniel S. L., Hsu T., Dean S. I., Drake H. L. Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui. J Bacteriol. 1990 Aug;172(8):4464–4471. doi: 10.1128/jb.172.8.4464-4471.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppenmeier U., Blaut M., Jussofie A., Gottschalk G. A methyl-CoM methylreductase system from methanogenic bacterium strain Gö 1 not requiring ATP for activity. FEBS Lett. 1988 Dec 5;241(1-2):60–64. doi: 10.1016/0014-5793(88)81031-7. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Ellermann J., Rospert S., Thauer R. K., Bokranz M., Klein A., Voges M., Berkessel A. Methyl-coenzyme-M reductase from Methanobacterium thermoautotrophicum (strain Marburg). Purity, activity and novel inhibitors. Eur J Biochem. 1989 Sep 1;184(1):63–68. doi: 10.1111/j.1432-1033.1989.tb14990.x. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978 Sep;190(1):351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Ljungdahl L. G. Isolation and characterization of an Fe,-S8 ferredoxin (ferredoxin II) from Clostridium thermoaceticum. J Bacteriol. 1982 Jul;151(1):328–333. doi: 10.1128/jb.151.1.328-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. L., Gorst C. M., Ragsdale S. W., Hoffman B. M. Characterization of the Ni-Fe-C complex formed by reaction of carbon monoxide with the carbon monoxide dehydrogenase from Clostridium thermoaceticum by Q-band ENDOR. Biochemistry. 1991 Jan 15;30(2):431–435. doi: 10.1021/bi00216a018. [DOI] [PubMed] [Google Scholar]
- Gorst C. M., Ragsdale S. W. Characterization of the NiFeCO complex of carbon monoxide dehydrogenase as a catalytically competent intermediate in the pathway of acetyl-coenzyme A synthesis. J Biol Chem. 1991 Nov 5;266(31):20687–20693. [PubMed] [Google Scholar]
- Hazzard J. T., Rong S. Y., Tollin G. Ionic strength dependence of the kinetics of electron transfer from bovine mitochondrial cytochrome c to bovine cytochrome c oxidase. Biochemistry. 1991 Jan 8;30(1):213–222. doi: 10.1021/bi00215a031. [DOI] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Pezacka E., Wood H. G. Acetate synthesis from carbon monoxide by Clostridium thermoaceticum. Purification of the corrinoid protein. J Biol Chem. 1984 Jul 25;259(14):8892–8897. [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. L., Kang D. S., Vitello L. B., Erman J. E. Cytochrome c peroxidase catalyzed oxidation of ferrocytochrome c by hydrogen peroxide: ionic strength dependence of the steady-state rate parameters. Biochemistry. 1990 Oct 2;29(39):9150–9159. doi: 10.1021/bi00491a008. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P. A., Münck E., Ragsdale S. W. CO dehydrogenase from Clostridium thermoaceticum. EPR and electrochemical studies in CO2 and argon atmospheres. J Biol Chem. 1990 Mar 5;265(7):3873–3879. [PubMed] [Google Scholar]
- Lindahl P. A., Ragsdale S. W., Münck E. Mössbauer study of CO dehydrogenase from Clostridium thermoaceticum. J Biol Chem. 1990 Mar 5;265(7):3880–3888. [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Lu W. P., Harder S. R., Ragsdale S. W. Controlled potential enzymology of methyl transfer reactions involved in acetyl-CoA synthesis by CO dehydrogenase and the corrinoid/iron-sulfur protein from Clostridium thermoaceticum. J Biol Chem. 1990 Feb 25;265(6):3124–3133. [PubMed] [Google Scholar]
- Lu W. P., Ragsdale S. W. Reductive activation of the coenzyme A/acetyl-CoA isotopic exchange reaction catalyzed by carbon monoxide dehydrogenase from Clostridium thermoaceticum and its inhibition by nitrous oxide and carbon monoxide. J Biol Chem. 1991 Feb 25;266(6):3554–3564. [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. The autotrophic pathway of acetogenic bacteria. Role of CO dehydrogenase disulfide reductase. J Biol Chem. 1986 Feb 5;261(4):1609–1615. [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. The synthesis of acetyl-CoA by Clostridium thermoaceticum from carbon dioxide, hydrogen, coenzyme A and methyltetrahydrofolate. Arch Microbiol. 1984 Jan;137(1):63–69. doi: 10.1007/BF00425809. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Ragsdale S. W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26(3-4):261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Lindahl P. A., Münck E. Mössbauer, EPR, and optical studies of the corrinoid/iron-sulfur protein involved in the synthesis of acetyl coenzyme A by Clostridium thermoaceticum. J Biol Chem. 1987 Oct 15;262(29):14289–14297. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. 13C and 61Ni isotope substitutions confirm the presence of a nickel (III)-carbon species in acetogenic CO dehydrogenases. Biochem Biophys Res Commun. 1983 Sep 15;115(2):658–665. doi: 10.1016/s0006-291x(83)80195-8. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Wood H. G. Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem. 1985 Apr 10;260(7):3970–3977. [PubMed] [Google Scholar]
- Ragsdale S. W., Wood H. G., Antholine W. E. Evidence that an iron-nickel-carbon complex is formed by reaction of CO with the CO dehydrogenase from Clostridium thermoaceticum. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6811–6814. doi: 10.1073/pnas.82.20.6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer S. E., Raybuck S. A., Orme-Johnson W. H., Walsh C. T. Kinetic characterization of the [3'-32P]coenzyme A/acetyl coenzyme A exchange catalyzed by a three-subunit form of the carbon monoxide dehydrogenase/acetyl-CoA synthase from Clostridium thermoaceticum. Biochemistry. 1989 May 30;28(11):4675–4680. doi: 10.1021/bi00437a025. [DOI] [PubMed] [Google Scholar]
- Raybuck S. A., Bastian N. R., Orme-Johnson W. H., Walsh C. T. Kinetic characterization of the carbon monoxide-acetyl-CoA (carbonyl group) exchange activity of the acetyl-CoA synthesizing CO dehydrogenase from Clostridium thermoaceticum. Biochemistry. 1988 Oct 4;27(20):7698–7702. doi: 10.1021/bi00420a019. [DOI] [PubMed] [Google Scholar]
- Roberts D. L., James-Hagstrom J. E., Garvin D. K., Gorst C. M., Runquist J. A., Baur J. R., Haase F. C., Ragsdale S. W. Cloning and expression of the gene cluster encoding key proteins involved in acetyl-CoA synthesis in Clostridium thermoaceticum: CO dehydrogenase, the corrinoid/Fe-S protein, and methyltransferase. Proc Natl Acad Sci U S A. 1989 Jan;86(1):32–36. doi: 10.1073/pnas.86.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Bobik T. A., Wolfe R. S. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Sep;170(9):3946–3952. doi: 10.1128/jb.170.9.3946-3952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundaram T., Kumar G. K., Wood H. G. Involvement of tryptophan residues at the coenzyme A binding site of carbon monoxide dehydrogenase from Clostridium thermoaceticum. Biochemistry. 1988 Aug 23;27(17):6499–6503. doi: 10.1021/bi00417a045. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram T., Ragsdale S. W., Wood H. G. Role of carbon monoxide dehydrogenase in acetate synthesis by the acetogenic bacterium, Acetobacterium woodii. Biofactors. 1988 Jul;1(2):147–152. [PubMed] [Google Scholar]
- Shanmugasundaram T., Wood H. G. Interaction of ferredoxin with carbon monoxide dehydrogenase from Clostridium thermoaceticum. J Biol Chem. 1992 Jan 15;267(2):897–900. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]