Abstract
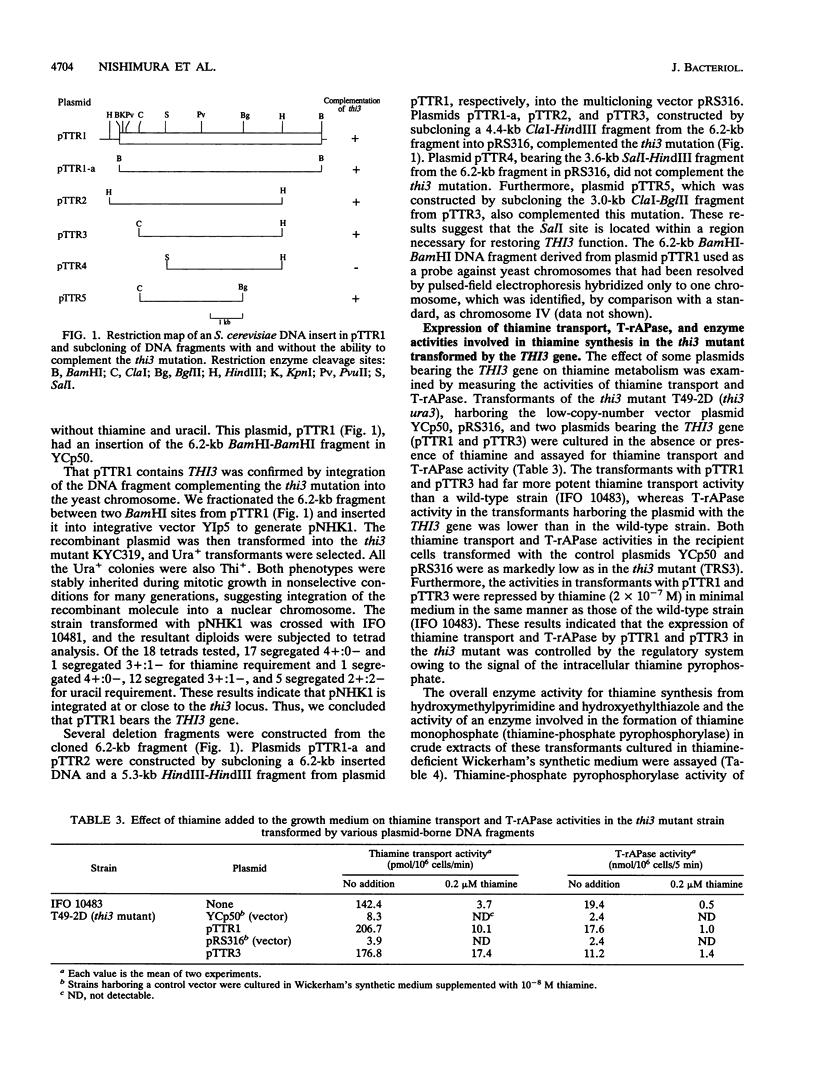
We have isolated a thiamine auxotrophic mutant carrying a recessive mutation which lacks the positive regulatory gene, THI3, which differs in the regulation of thiamine transport from the THI2 (PHO6) gene described previously (Y. Kawasaki, K. Nosaka, Y. Kaneko, H. Nishimura, and A. Iwashima, J. Bacteriol. 172:6145-6147, 1990) for expression of thiamine metabolism in Saccharomyces cerevisiae. The mutant (thi3) had a markedly reduced thiamine transport system as well as reduced activity of thiamine-repressible acid phosphatase and of several enzymes for thiamine synthesis from 2-methyl-4-amino-5-hydroxymethylpyrimidine and 4-methyl-5-beta-hydroxyethylthiazole. These results suggest that thiamine metabolism in S. cerevisiae is subject to two positive regulatory genes, THI2 (PHO6) and THI3. We have also isolated a hybrid plasmid, pTTR1, containing a 6.2-kb DNA fragment from an S. cerevisiae genomic library which complements thiamine auxotrophy in the thi3 mutant. This gene was localized on a 3.0-kb ClaI-BglII fragment in the subclone pTTR5. Complementation of the activities for thiamine metabolism in the thi3 mutant transformed by some plasmids with the THI3 gene was also examined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991 Jun;11(6):3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMIENER G. W., BROWN G. M. The biosynthesis of thiamine. 2. Fractionation of enzyme system and identification of thiazole monophosphate and thiamine monophosphate as intermediates. J Biol Chem. 1960 Aug;235:2411–2417. [PubMed] [Google Scholar]
- Hawthorne D C, Mortimer R K. Chromosome Mapping in Saccharomyces: Centromere-Linked Genes. Genetics. 1960 Aug;45(8):1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima A., Nishino H., Nose Y. Carrier-mediated transport of thiamine in baker's yeast. Biochim Biophys Acta. 1973 Dec 13;330(2):222–234. doi: 10.1016/0005-2736(73)90227-7. [DOI] [PubMed] [Google Scholar]
- Iwashima A., Wakabayashi Y., Nose Y. Thiamine transport mutants of Saccharomyces cerevisiae. Biochim Biophys Acta. 1975 Dec 1;413(2):243–247. doi: 10.1016/0005-2736(75)90108-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Nosaka K., Kaneko Y., Nishimura H., Iwashima A. Regulation of thiamine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1990 Oct;172(10):6145–6147. doi: 10.1128/jb.172.10.6145-6147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemire J. M., Willcocks T., Halvorson H. O., Bostian K. A. Regulation of repressible acid phosphatase gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Aug;5(8):2131–2141. doi: 10.1128/mcb.5.8.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D., Contopoulou C. R., Kans J. A. Genetic map of Saccharomyces cerevisiae, edition 10. Yeast. 1989 Sep-Oct;5(5):321–403. doi: 10.1002/yea.320050503. [DOI] [PubMed] [Google Scholar]
- NOSE Y., UEDA K., KAWASAKI T. Enzymic synthesis of thiamine. Biochim Biophys Acta. 1959 Jul;34:277–279. doi: 10.1016/0006-3002(59)90270-7. [DOI] [PubMed] [Google Scholar]
- NOSE Y., UEDA K., KAWASAKI T., IWASHIMA A., FUJITA T. Enzymatic synthesis of thiamine. II. The thiamine synthesis from pyrimidine and thiazole phosphates and the enzymatic synthesis of pyrimidine mono- and diphosphate and thiazole monophosphate. J Vitaminol (Kyoto) 1961 Jun 10;7:98–114. [PubMed] [Google Scholar]
- Nishimura H., Kawasaki Y., Kaneko Y., Nosaka K., Iwashima A. Cloning and characteristics of a positive regulatory gene, THI2 (PHO6), of thiamin biosynthesis in Saccharomyces cerevisiae. FEBS Lett. 1992 Feb 3;297(1-2):155–158. doi: 10.1016/0014-5793(92)80349-l. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kawasaki Y., Nosaka K., Kaneko Y., Iwashima A. A constitutive thiamine metabolism mutation, thi80, causing reduced thiamine pyrophosphokinase activity in Saccharomyces cerevisiae. J Bacteriol. 1991 Apr;173(8):2716–2719. doi: 10.1128/jb.173.8.2716-2719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka K. High affinity of acid phosphatase encoded by PHO3 gene in Saccharomyces cerevisiae for thiamin phosphates. Biochim Biophys Acta. 1990 Feb 9;1037(2):147–154. doi: 10.1016/0167-4838(90)90160-h. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Tamai Y., Toh-e A., Oshima Y. Regulation of inorganic phosphate transport systems in Saccharomyces cerevisiae. J Bacteriol. 1985 Nov;164(2):964–968. doi: 10.1128/jb.164.2.964-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Kakimoto S. Genes coding for the structure of the acid phosphatases in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Dec 30;143(1):65–70. doi: 10.1007/BF00269421. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Kakimoto S., Oshima Y. Two new genes controlling the constitutive acid phosphatase synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Nov 3;141(1):81–83. doi: 10.1007/BF00332380. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Ogawa N., Oshima Y. Function of the PHO regulatory genes for repressible acid phosphatase synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1989 May;217(1):40–46. doi: 10.1007/BF00330940. [DOI] [PubMed] [Google Scholar]



