Abstract
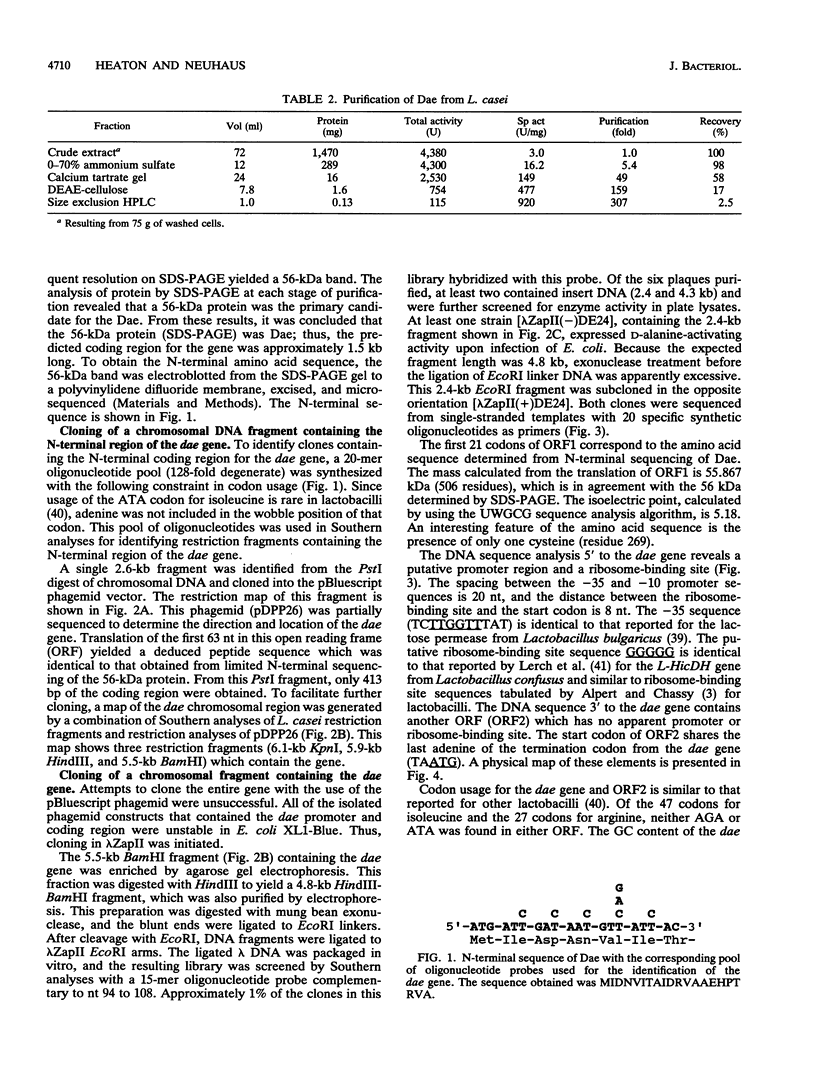
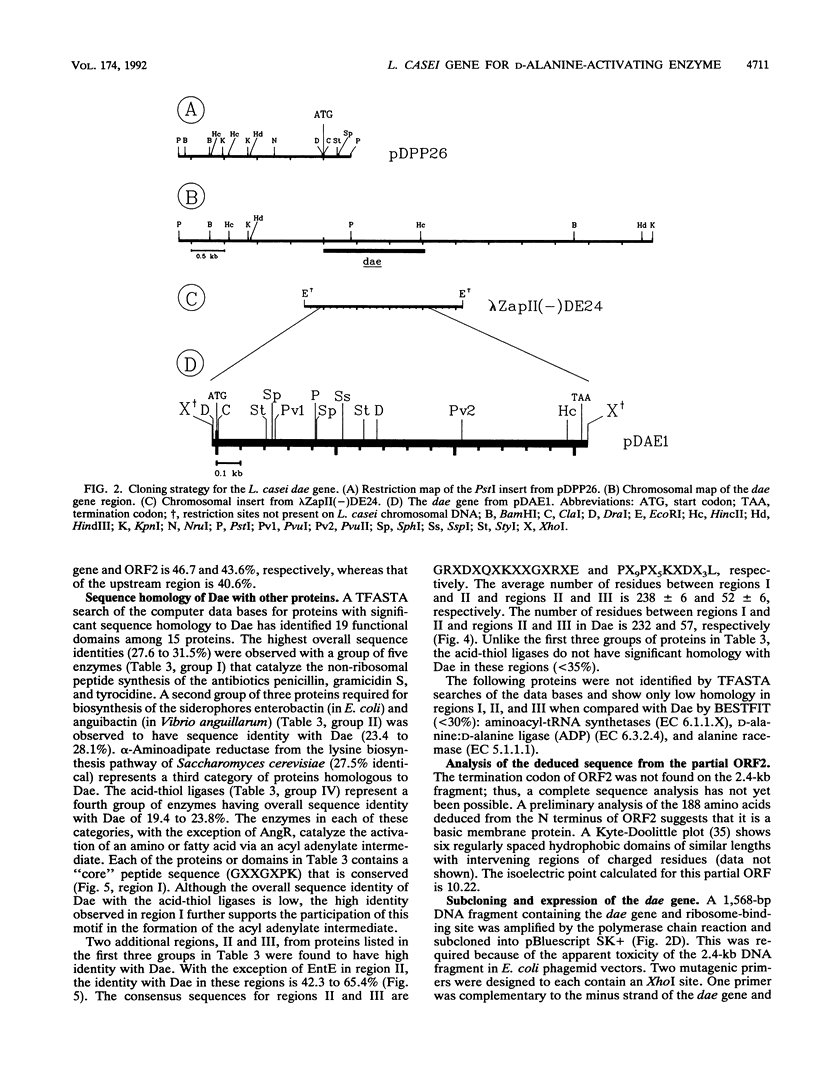
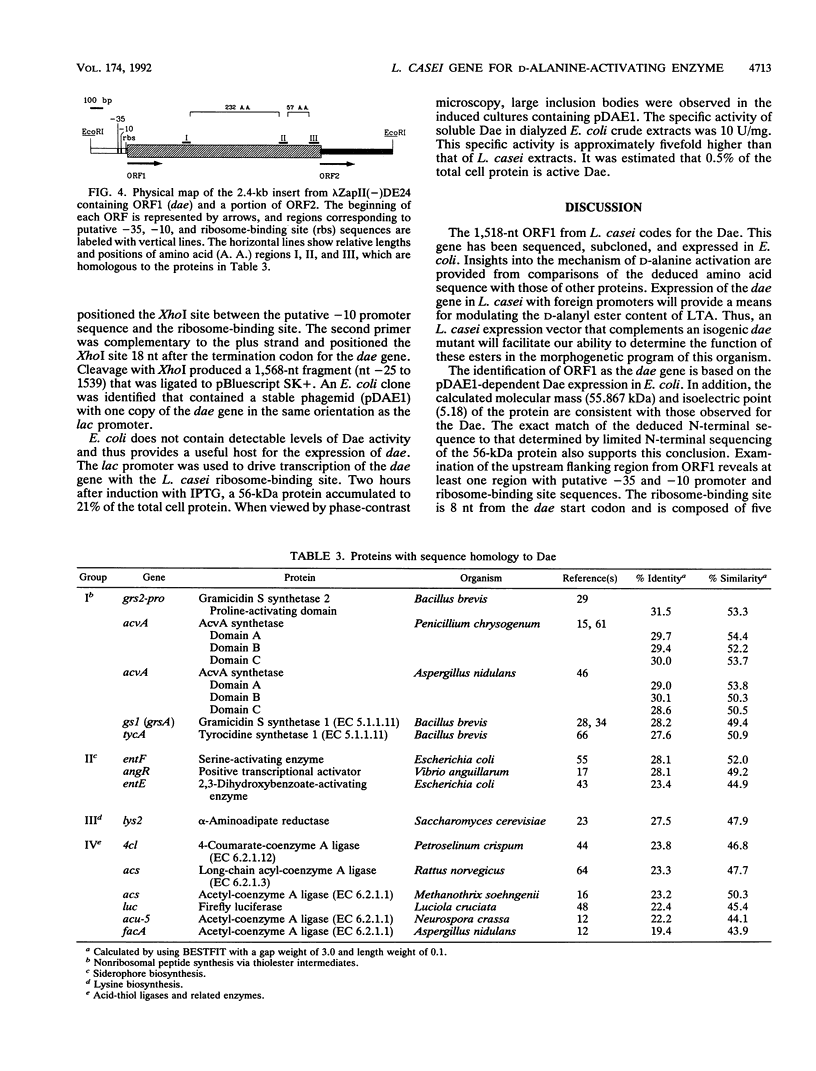
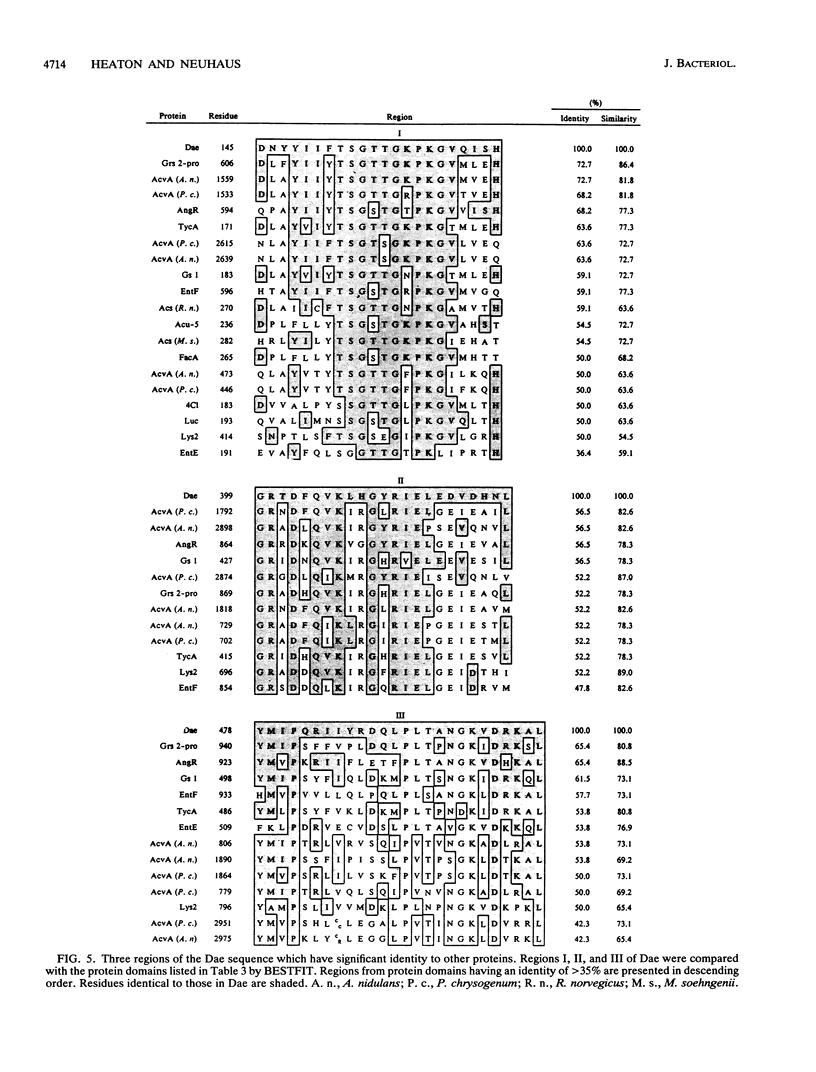
The D-alanine-activating enzyme (Dae; EC 6.3.2.4) encoded by the dae gene from Lactobacillus casei ATCC 7469 is a cytosolic protein essential for the formation of the D-alanyl esters of membrane-bound lipoteichoic acid. The gene has been cloned, sequenced, and expressed in Escherichia coli, an organism which does not possess Dae activity. The open reading frame is 1,518 nucleotides and codes for a protein of 55.867 kDa, a value in agreement with the 56 kDa obtained by electrophoresis. A putative promoter and ribosome-binding site immediately precede the dae gene. A second open reading frame contiguous with the dae gene has also been partially sequenced. The organization of these genetic elements suggests that more than one enzyme necessary for the biosynthesis of D-alanyl-lipoteichoic acid may be present in this operon. Analysis of the amino acid sequence deduced from the dae gene identified three regions with significant homology to proteins in the following groups of ATP-utilizing enzymes: (i) the acid-thiol ligases, (ii) the activating enzymes for the biosynthesis of enterobactin, and (iii) the synthetases for tyrocidine, gramicidin S, and penicillin. From these comparisons, a common motif (GXXGXPK) has been identified that is conserved in the 19 protein domains analyzed. This motif may represent the phosphate-binding loop of an ATP-binding site for this class of enzymes. A DNA fragment (1,568 nucleotides) containing the dae gene and its putative ribosome-binding site has been subcloned and expressed in E. coli. Approximately 0.5% of the total cell protein is active Dae, whereas 21% is in the form of inclusion bodies. The isolation of this minimal fragment without a native promoter sequence provides the basis for designing a genetic system for modulating the D-alanine ester content of lipoteichoic acid.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhrem A. A., Drozhdenyuk A. P. Calcium tartrate gel. Anal Biochem. 1989 May 15;179(1):86–89. doi: 10.1016/0003-2697(89)90205-4. [DOI] [PubMed] [Google Scholar]
- Alpert C. A., Chassy B. M. Molecular cloning and DNA sequence of lacE, the gene encoding the lactose-specific enzyme II of the phosphotransferase system of Lactobacillus casei. Evidence that a cysteine residue is essential for sugar phosphorylation. J Biol Chem. 1990 Dec 25;265(36):22561–22568. [PubMed] [Google Scholar]
- Alpert C. A., Chassy B. M. Molecular cloning and nucleotide sequence of the factor IIIlac gene of Lactobacillus casei. Gene. 1988;62(2):277–288. doi: 10.1016/0378-1119(88)90565-3. [DOI] [PubMed] [Google Scholar]
- Andrews J., Clore G. M., Davies R. W., Gronenborn A. M., Gronenborn B., Kalderon D., Papadopoulos P. C., Schäfer S., Sims P. F., Stancombe R. Nucleotide sequence of the dihydrofolate reductase gene of methotrexate-resistant Lactobacillus casei. Gene. 1985;35(1-2):217–222. doi: 10.1016/0378-1119(85)90174-x. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim Biophys Acta. 1973 Feb 16;291(3):629–634. doi: 10.1016/0005-2736(73)90468-9. [DOI] [PubMed] [Google Scholar]
- BADDILEY J., NEUHAUS F. C. The enzymic activation of D-alanine. Biochem J. 1960 Jun;75:579–587. doi: 10.1042/bj0750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D. Equipping the laboratory. Methods Enzymol. 1987;152:3–20. doi: 10.1016/0076-6879(87)52004-3. [DOI] [PubMed] [Google Scholar]
- Brautigan V. M., Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei: D-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981 Apr;146(1):239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: characterization of ester-linked D-alanine in the in vitro-synthesized product. J Bacteriol. 1980 Jul;143(1):293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerton I. F., Fincham J. R., Sandeman R. A., Hynes M. J. Comparison and cross-species expression of the acetyl-CoA synthetase genes of the Ascomycete fungi, Aspergillus nidulans and Neurospora crassa. Mol Microbiol. 1990 Mar;4(3):451–460. doi: 10.1111/j.1365-2958.1990.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Gronenborn A. M. Molecular cloning of the gene for dihydrofolate reductase from Lactobacillus casei. Gene. 1982 Feb;17(2):229–233. doi: 10.1016/0378-1119(82)90077-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez B., Gutiérrez S., Barredo J. L., van Solingen P., van der Voort L. H., Martín J. F. The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the alpha-aminoadipyl-cysteinyl-valine synthetase and linkage to the pcbC and penDE genes. J Biol Chem. 1990 Sep 25;265(27):16358–16365. [PubMed] [Google Scholar]
- Eggen R. I., Geerling A. C., Boshoven A. B., de Vos W. M. Cloning, sequence analysis, and functional expression of the acetyl coenzyme A synthetase gene from Methanothrix soehngenii in Escherichia coli. J Bacteriol. 1991 Oct;173(20):6383–6389. doi: 10.1128/jb.173.20.6383-6389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Mikesell P., Actis L. A., Crosa J. H. A regulatory gene, angR, of the iron uptake system of Vibrio anguillarum: similarity with phage P22 cro and regulation by iron. Gene. 1990 Jan 31;86(1):45–51. doi: 10.1016/0378-1119(90)90112-5. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Fischer W., Rösel P., Koch H. U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981 May;146(2):467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Rösel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 1980 Oct 6;119(2):224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- Fleig U. N., Pridmore R. D., Philippsen P. Construction of LYS2 cartridges for use in genetic manipulations of Saccharomyces cerevisiae. Gene. 1986;46(2-3):237–245. doi: 10.1016/0378-1119(86)90408-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hori K., Yamamoto Y., Minetoki T., Kurotsu T., Kanda M., Miura S., Okamura K., Furuyama J., Saito Y. Molecular cloning and nucleotide sequence of the gramicidin S synthetase 1 gene. J Biochem. 1989 Oct;106(4):639–645. doi: 10.1093/oxfordjournals.jbchem.a122909. [DOI] [PubMed] [Google Scholar]
- Hori K., Yamamoto Y., Tokita K., Saito F., Kurotsu T., Kanda M., Okamura K., Furuyama J., Saito Y. The nucleotide sequence for a proline-activating domain of gramicidin S synthetase 2 gene from Bacillus brevis. J Biochem. 1991 Jul;110(1):111–119. doi: 10.1093/oxfordjournals.jbchem.a123528. [DOI] [PubMed] [Google Scholar]
- Hughes A. H., Hancock I. C., Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973 Jan;132(1):83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELEMEN M. V., BADDILEY J. Structure of the intracellular glycerol teichoic acid from Lactobacillus casei A.T.C.C. 7469. Biochem J. 1961 Aug;80:246–254. doi: 10.1042/bj0800246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Biosynthesis of peptide antibiotics. Annu Rev Microbiol. 1987;41:259–289. doi: 10.1146/annurev.mi.41.100187.001355. [DOI] [PubMed] [Google Scholar]
- Koch H. U., Fischer W., Fiedler F. Influence of alanine ester and glycosyl substitution on the lipoteichoic acid carrier activity of lipoteichoic acids. J Biol Chem. 1982 Aug 25;257(16):9473–9479. [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acids. Biochem J. 1975 Dec;151(3):671–676. doi: 10.1042/bj1510671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. J., Hansen J. B., Jagusztyn-Krynicka E. K., Chassy B. M. Cloning and expression of the beta-D-phosphogalactoside galactohydrolase gene of Lactobacillus casei in Escherichia coli K-12. J Bacteriol. 1982 Dec;152(3):1138–1146. doi: 10.1128/jb.152.3.1138-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong-Morgenthaler P., Zwahlen M. C., Hottinger H. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J Bacteriol. 1991 Mar;173(6):1951–1957. doi: 10.1128/jb.173.6.1951-1957.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch H. P., Blöcker H., Kallwass H., Hoppe J., Tsai H., Collins J. Cloning, sequencing and expression in Escherichia coli of the D-2-hydroxyisocaproate dehydrogenase gene of Lactobacillus casei. Gene. 1989 May 15;78(1):47–57. doi: 10.1016/0378-1119(89)90313-2. [DOI] [PubMed] [Google Scholar]
- Lerch H. P., Frank R., Collins J. Cloning, sequencing and expression of the L-2-hydroxyisocaproate dehydrogenase-encoding gene of Lactobacillus confusus in Escherichia coli. Gene. 1989 Nov 30;83(2):263–270. doi: 10.1016/0378-1119(89)90112-1. [DOI] [PubMed] [Google Scholar]
- Linzer R., Neuhaus F. C. Biosynthesis of membrane teichoic acid. A role of the D-alanine-activating enzyme. J Biol Chem. 1973 May 10;248(9):3196–3201. [PubMed] [Google Scholar]
- Liu J., Duncan K., Walsh C. T. Nucleotide sequence of a cluster of Escherichia coli enterobactin biosynthesis genes: identification of entA and purification of its product 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase. J Bacteriol. 1989 Feb;171(2):791–798. doi: 10.1128/jb.171.2.791-798.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoya E., Hoffmann H., Douglas C., Schulz W., Scheel D., Hahlbrock K. Primary structures and catalytic properties of isoenzymes encoded by the two 4-coumarate: CoA ligase genes in parsley. Eur J Biochem. 1988 Oct 1;176(3):661–667. doi: 10.1111/j.1432-1033.1988.tb14328.x. [DOI] [PubMed] [Google Scholar]
- MacArthur A. E., Archibald A. R. Effect of culture pH on the D-alanine ester content of lipoteichoic acid in Staphylococcus aureus. J Bacteriol. 1984 Nov;160(2):792–793. doi: 10.1128/jb.160.2.792-793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe A. P., van Liempt H., Palissa H., Unkles S. E., Riach M. B., Pfeifer E., von Döhren H., Kinghorn J. R. Delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. Molecular characterization of the acvA gene encoding the first enzyme of the penicillin biosynthetic pathway. J Biol Chem. 1991 Jul 5;266(19):12646–12654. [PubMed] [Google Scholar]
- Masuda T., Tatsumi H., Nakano E. Cloning and sequence analysis of cDNA for luciferase of a Japanese firefly, Luciola cruciata. Gene. 1989 Apr 30;77(2):265–270. doi: 10.1016/0378-1119(89)90074-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Natori Y., Kano Y., Imamoto F. Nucleotide sequences and genomic constitution of five tryptophan genes of Lactobacillus casei. J Biochem. 1990 Feb;107(2):248–255. doi: 10.1093/oxfordjournals.jbchem.a123034. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C., Linzer R., Reusch V. M., Jr Biosynthesis of membrane teichoic acid: role of the D-alanine-activating enzyme and D-alanine: membrane acceptor ligase. Ann N Y Acad Sci. 1974 May 10;235(0):502–518. doi: 10.1111/j.1749-6632.1974.tb43287.x. [DOI] [PubMed] [Google Scholar]
- Ntamere A. S., Taron D. J., Neuhaus F. C. Assembly of D-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the D-alanyl ester content of this amphiphile. J Bacteriol. 1987 Apr;169(4):1702–1711. doi: 10.1128/jb.169.4.1702-1711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E. V., Chassy B. M. Nucleotide sequence of the beta-D-phosphogalactoside galactohydrolase gene of Lactobacillus casei: comparison to analogous pbg genes of other gram-positive organisms. Gene. 1988;62(2):263–276. doi: 10.1016/0378-1119(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Neuhaus F. C. D-Alanine: membrane acceptor ligase from Lactobacillus casei. J Biol Chem. 1971 Oct 25;246(20):6136–6143. [PubMed] [Google Scholar]
- Rusnak F., Sakaitani M., Drueckhammer D., Reichert J., Walsh C. T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991 Mar 19;30(11):2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salinas P. C., Tolmasky M. E., Crosa J. H. Regulation of the iron uptake system in Vibrio anguillarum: evidence for a cooperative effect between two transcriptional activators. Proc Natl Acad Sci U S A. 1989 May;86(10):3529–3533. doi: 10.1073/pnas.86.10.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Earl A. J., Turner G. The multifunctional peptide synthetase performing the first step of penicillin biosynthesis in Penicillium chrysogenum is a 421,073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J. 1990 Sep;9(9):2743–2750. doi: 10.1002/j.1460-2075.1990.tb07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe I. C., Shaw N. Atypical lipoteichoic acids of gram-positive bacteria. J Bacteriol. 1991 Nov;173(22):7065–7069. doi: 10.1128/jb.173.22.7065-7069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kawarabayasi Y., Kondo J., Abe T., Nishikawa K., Kimura S., Hashimoto T., Yamamoto T. Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem. 1990 May 25;265(15):8681–8685. [PubMed] [Google Scholar]
- Toy J., Bognar A. L. Cloning and expression of the gene encoding Lactobacillus casei folylpoly-gamma-glutamate synthetase in Escherichia coli and determination of its primary structure. J Biol Chem. 1990 Feb 15;265(5):2492–2499. [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]



