Abstract
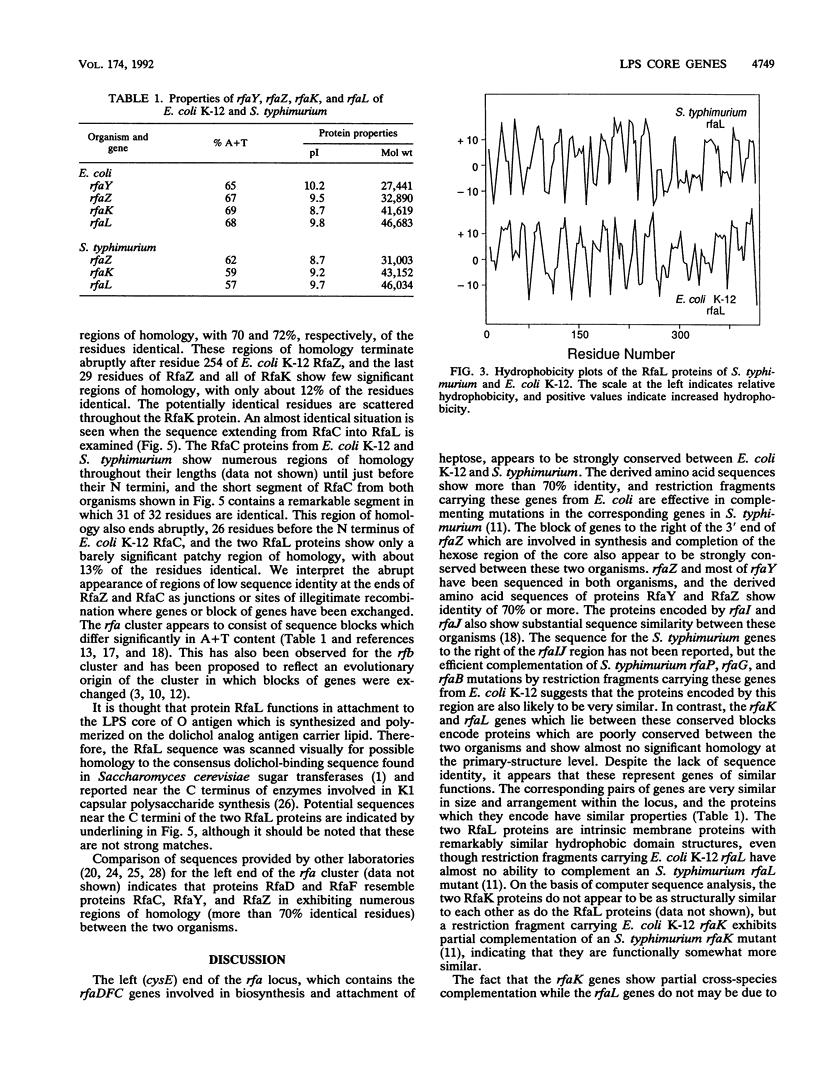
Analysis of the sequence of a 4.3-kb region downstream of rfaJ revealed four genes. The first two of these, which encode proteins of 27,441 and 32,890 Da, were identified as rfaY and rfaZ by homology of the derived protein sequences of their products to the products of similar genes of Salmonella typhimurium. The amino acid sequences of proteins RfaY and RfaZ showed, respectively, 70 and 72% identity. Genes 3 and 4 were identified as rfaK and rfaL on the basis of size and position, but the derived amino acid sequences of the products of these genes showed very little similarity (about 12% identity) between Escherichia coli K-12 and S. typhimurium. The next gene in the cluster, rfaC, encodes a product which also shows strong protein sequence homology between E. coli K-12 and S. typhimurium, as do the rfaF and rfaD genes which lie beyond it. Thus, the rfa gene cluster appears to consist of two blocks of genes which are conserved flanking a central region of two genes which are not conserved between these species. Although the RfaL protein sequence is not conserved, hydropathy plots of the two RfaL species are nearly identical and indicate that this is a typical integral membrane protein with 10 or more potential transmembrane domains. We noted the similarity of the structure of the rfa gene cluster to that of the rfb gene cluster, which has now been sequenced in several Salmonella serovars. The rfb cluster also contains a gene which lies within a central nonconserved region and encodes an integral membrane protein similar to protein RfaL. We speculate that protein RfaL may interact in a strain- or species-specific way with one or more Rfb proteins in the expression of surface O antigen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright C. F., Orlean P., Robbins P. W. A 13-amino acid peptide in three yeast glycosyltransferases may be involved in dolichol recognition. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7366–7369. doi: 10.1073/pnas.86.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Creeger E. S., Schulte T., Rothfield L. I. Regulation of membrane glycosyltransferases by the sfrB and rfaH genes of Escherichia coli and Salmonella typhimurium. J Biol Chem. 1984 Mar 10;259(5):3064–3069. [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg A. A., Lindberg B., Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981 Apr;115(3):571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- Jiang X. M., Neal B., Santiago F., Lee S. J., Romana L. K., Reeves P. R. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol Microbiol. 1991 Mar;5(3):695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Liu D., Verma N. K., Romana L. K., Reeves P. R. Relationships among the rfb regions of Salmonella serovars A, B, and D. J Bacteriol. 1991 Aug;173(15):4814–4819. doi: 10.1128/jb.173.15.4814-4819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan P. R., Kadam S. K., Sanderson K. E. Cloning, characterization, and DNA sequence of the rfaLK region for lipopolysaccharide synthesis in Salmonella typhimurium LT2. J Bacteriol. 1991 Nov;173(22):7151–7163. doi: 10.1128/jb.173.22.7151-7163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990 Feb;172(2):1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Kloser A. W., Schnaitman C. A., Stein M. A., Gottesman S., Gibson B. W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992 Apr;174(8):2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E., Parker C. T., Schnaitman C. A. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J Bacteriol. 1992 Jul;174(14):4736–4745. doi: 10.1128/jb.174.14.4736-4745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E., Schnaitman C. A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A., Kadam S. K., Sanderson K. E. Cloning and analysis of the sfrB (sex factor repression) gene of Escherichia coli K-12. J Bacteriol. 1986 May;166(2):651–657. doi: 10.1128/jb.166.2.651-657.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Parker C. T., Klena J. D., Pradel E. L., Pearson N. B., Sanderson K. E., MacClachlan P. R. Physical maps of the rfa loci of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7410–7411. doi: 10.1128/jb.173.23.7410-7411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen S. M., Wrona T. J., Vimr E. R. Functional analysis of the sialyltransferase complexes in Escherichia coli K1 and K92. J Bacteriol. 1992 Feb;174(4):1099–1108. doi: 10.1128/jb.174.4.1099-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Forrest B., Morona R., Attridge S. R., LaBrooy J., Tall B. D., Reymann M., Rowley D., Levine M. M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990 Jun;58(6):1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



