Abstract
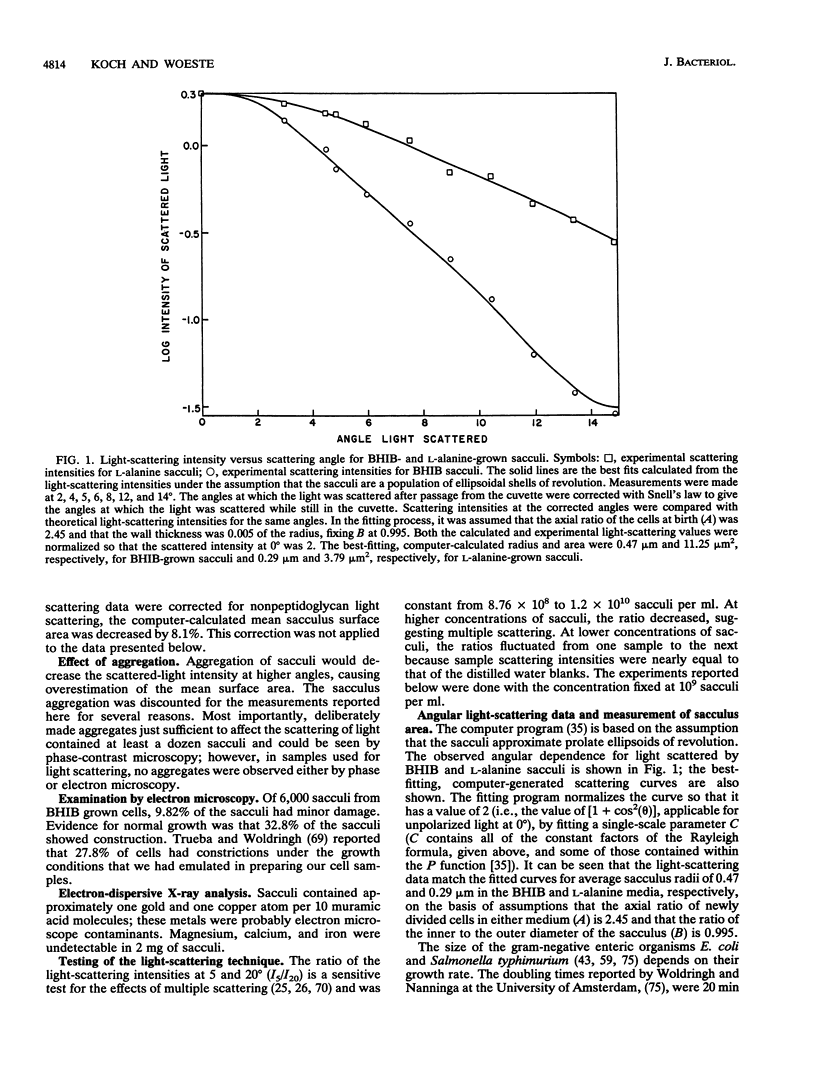
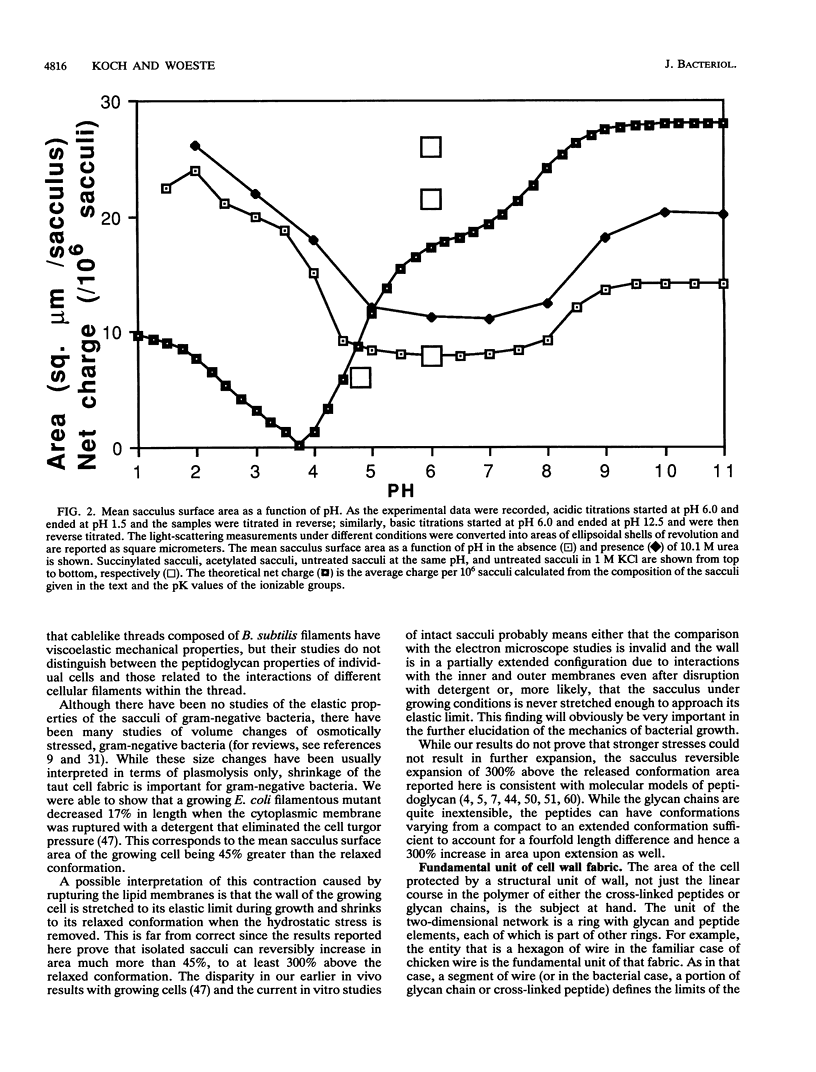
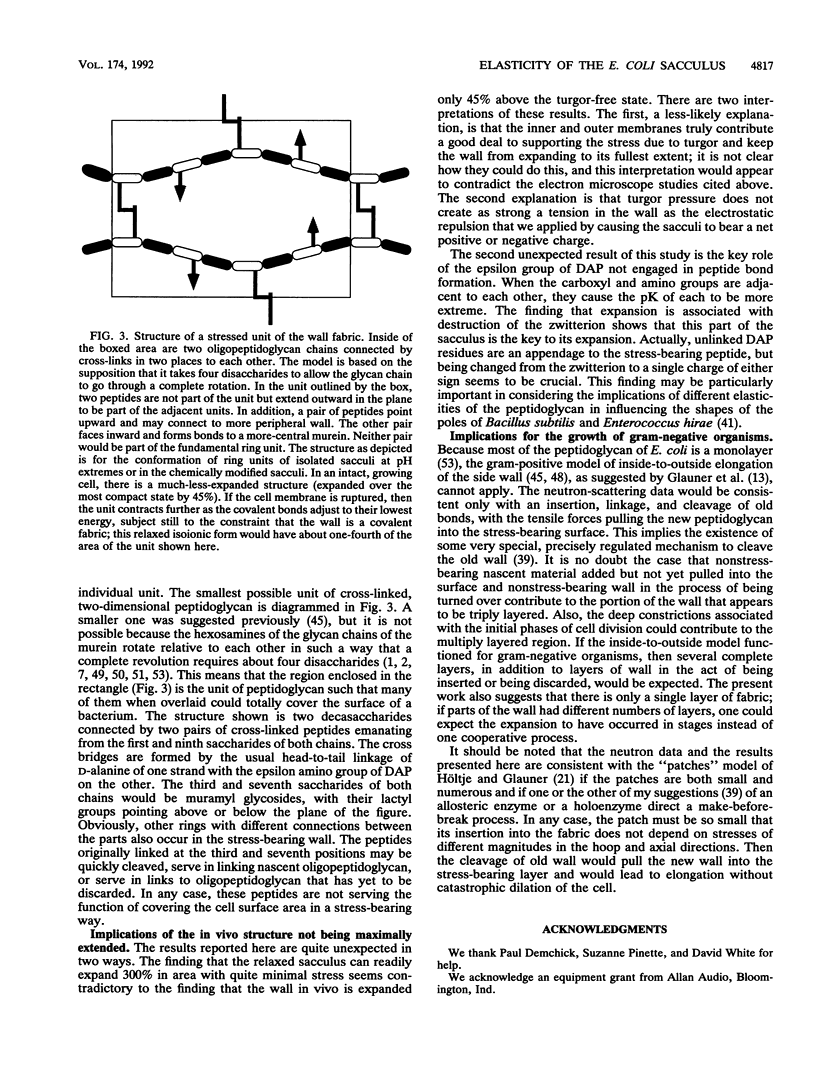
Preparations of purified peptidoglycan of Escherichia coli (i.e., sacculi) were studied by low-angle laser light scattering. Control experiments and theoretical calculations based on the Rayleigh-Gans theory showed that the mean sacculus surface area could be accurately inferred from measurements with our apparatus by using computer routines developed previously. Large changes in the mean saccular surface area resulted from alterations in the stress caused by varying the net charge on the sacculi. The net charge was affected by altering the suspending medium pH, causing carboxyl and amino groups in the peptidoglycan to gain or lose protons, or by acetylation or succinylation of the amino groups. A preponderance of either plus or minus charges caused an expansion of the mean sacculus surface area. The largest increase in area probably represents the elastic limit of the peptidoglycan and was 300% above the area of isoionic sacculi. This degree of expansion is consistent with possible conformations of the intact peptidoglycan structure without necessitating rupture of the wall fabric. Our findings concerning saccular elasticity provide support for the surface stress theory. It provides a mechanism so that bacteria can grow and divide while maintaining turgor pressure, without the necessity of having and using proteins to do the mechanical work.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnickel G., Labischinski H., Bradaczek H., Giesbrecht P. Conformational energy calculation on the peptide part of murein. Eur J Biochem. 1979 Mar 15;95(1):157–165. doi: 10.1111/j.1432-1033.1979.tb12950.x. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Burge R. E., Adams R., Balyuzi H. H., Reaveley D. A. Structure of the peptidoglycan of bacterial cell wassl. II. J Mol Biol. 1977 Dec 25;117(4):955–974. doi: 10.1016/s0022-2836(77)80007-7. [DOI] [PubMed] [Google Scholar]
- Burge R. E., Fowler A. G., Reaveley D. A. Structure of the peptidogylcan of bacterial cell walls. I. J Mol Biol. 1977 Dec 25;117(4):927–953. doi: 10.1016/s0022-2836(77)80006-5. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Matthews T. H., Streips U. N. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980 Jul;143(1):471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Harz H., Burgdorf K., Höltje J. V. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal Biochem. 1990 Oct;190(1):120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tuomanen E. I. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J Gen Microbiol. 1991 Mar;137(3):441–454. doi: 10.1099/00221287-137-3-441. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Morris S. J., Striker G., Schultens H. A., Digweed M., Arndt-Jovin D. J. Automatic sizing and separation of particles by ratios of light scattering intensities. J Histochem Cytochem. 1976 Jan;24(1):269–283. doi: 10.1177/24.1.1254922. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Additional arguments for the key role of "smart" autolysins in the enlargement of the wall of gram-negative bacteria. Res Microbiol. 1990 Jun;141(5):529–541. doi: 10.1016/0923-2508(90)90017-k. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Biophysics of bacterial walls viewed as stress-bearing fabric. Microbiol Rev. 1988 Sep;52(3):337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. The variable T model for gram-negative morphology. J Gen Microbiol. 1984 Sep;130(9):2325–2338. doi: 10.1099/00221287-130-9-2325. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Differences in the formation of poles of Enterococcus and Bacillus. J Theor Biol. 1992 Jan 21;154(2):205–217. doi: 10.1016/s0022-5193(05)80403-5. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Ehrenfeld E. Th size and shape of bacteria by light scattering measurements. Biochim Biophys Acta. 1968 Sep 3;165(2):262–273. doi: 10.1016/0304-4165(68)90054-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981 Mar;123(1):151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. The role of surface stress in the morphology of microbes. J Gen Microbiol. 1982 May;128(5):927–945. doi: 10.1099/00221287-128-5-927. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Lane S. L., Miller J. A., Nickens D. G. Contraction of filaments of Escherichia coli after disruption of cell membrane by detergent. J Bacteriol. 1987 May;169(5):1979–1984. doi: 10.1128/jb.169.5.1979-1984.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. On the growth and form of Escherichia coli. J Gen Microbiol. 1982 Nov;128(11):2527–2539. doi: 10.1099/00221287-128-11-2527. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Shrinkage of growing Escherichia coli cells by osmotic challenge. J Bacteriol. 1984 Sep;159(3):919–924. doi: 10.1128/jb.159.3.919-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory for the case of Escherichia coli: the paradoxes of gram-negative growth. Res Microbiol. 1990 Jan;141(1):119–130. doi: 10.1016/0923-2508(90)90103-w. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Theory of the angular dependence of light scattered by bacteria and similar-sized biological objects. J Theor Biol. 1968 Jan;18(1):133–156. doi: 10.1016/0022-5193(68)90174-4. [DOI] [PubMed] [Google Scholar]
- LEUTGEB W., WEIDEL W. UBER EIN IN COLI-ZELLWANDPRAEPARATEN ZURUECKGEHALTENES GLYKOGEN. Z Naturforsch B. 1963 Dec;18:1060–1062. [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Bradaczek H., Giesbrecht P. On the secondary and tertiary structure of murein. Low and medium-angle X-ray evidence against chitin-based conformations of bacterial peptidoglycan. Eur J Biochem. 1979 Mar 15;95(1):147–155. doi: 10.1111/j.1432-1033.1979.tb12949.x. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Naumann D., Keller P. Conformational and topological aspects of the three-dimensional architecture of bacterial peptidoglycan. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):45–50. doi: 10.1016/s0769-2609(85)80020-x. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Goodell E. W., Goodell A., Hochberg M. L. Direct proof of a "more-than-single-layered" peptidoglycan architecture of Escherichia coli W7: a neutron small-angle scattering study. J Bacteriol. 1991 Jan;173(2):751–756. doi: 10.1128/jb.173.2.751-756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney P. F., Dean P. N. The small angle light scattering of biological cells. Theoretical considerations. Biophys J. 1970 Aug;10(8):764–772. doi: 10.1016/S0006-3495(70)86334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldmixon E. H., Glauser S., Higgins M. L. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers. 1974;13(10):2037–2060. doi: 10.1002/bip.1974.360131008. [DOI] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Coccal cell-wall compactness and the swelling action of denaturants. Can J Microbiol. 1972 May;18(5):623–629. doi: 10.1139/m72-099. [DOI] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T., Kohlrausch U., Burgdorf K., Höltje J. V. Murein chemistry of cell division in Escherichia coli. Res Microbiol. 1991 Feb-Apr;142(2-3):325–332. doi: 10.1016/0923-2508(91)90048-f. [DOI] [PubMed] [Google Scholar]
- Shively J. M. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28(0):167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Thwaites J. J., Mendelson N. H. Mechanical behaviour of bacterial cell walls. Adv Microb Physiol. 1991;32:173–222. doi: 10.1016/s0065-2911(08)60008-9. [DOI] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Woldringh C. L., Nanninga N. Amount of peptidoglycan in cell walls of gram-negative bacteria. J Bacteriol. 1991 Dec;173(23):7684–7691. doi: 10.1128/jb.173.23.7684-7691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]


