Abstract
Penicillin-binding proteins (PBPs), although characterized from several organisms, have so far not been studied in mycobacteria. The present study is the first characterization of a PBP from Mycobacterium smegmatis. The PBP was purified by solubilization of the membranes with Triton X-100 and successive chromatography of the solubilized proteins on ampicillin-linked CH Sepharose 4B and DE-52. The purified PBP (M(r), 49,500) catalyzed a model transpeptidase reaction with the tripeptide acetyl2-L-Lys-D-Ala-D-Ala as the substrate and Gly-Gly as the acceptor. The transpeptidase activity was inhibited by 50% at a benzylpenicillin concentration of 1.8 x 10(-7) M, which was similar to the concentration (1.1 x 10(-7) M) of benzylpenicillin required to saturate to 50% this PBP. Of several antibiotics tested, the concentration of antibiotic required to inhibit [35S]penicillin binding by 90% was found to be the lowest for cefoxitin and Sch 34343.
Full text
PDF

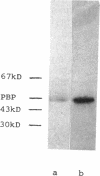
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Das P. R., Pramanik B. N., Girijavallabhan V. M., Ganguly A. K. Ionization of penem beta-lactam antibiotics using thermospray (filament-on) mass spectrometry. J Antibiot (Tokyo) 1988 Sep;41(9):1268–1271. doi: 10.7164/antibiotics.41.1268. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Extensive re-modelling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989 Jan;3(1):95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Woodford N., Johnson A. P., George R. C., Spratt B. G. Penicillin-resistant viridans streptococci have obtained altered penicillin-binding protein genes from penicillin-resistant strains of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5858–5862. doi: 10.1073/pnas.87.15.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun H. M., Yapo A., Petit J. F. DD-Carboxypeptidase activity of membrane fragments of Mycobacterium smegmatis. Enzymatic properties and sensitivity to beta-lactam antibiotics. Eur J Biochem. 1978 May;86(1):97–103. doi: 10.1111/j.1432-1033.1978.tb12288.x. [DOI] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Bacterial active-site serine penicillin-interactive proteins and domains: mechanism, structure, and evolution. Rev Infect Dis. 1988 Jul-Aug;10(4):726–732. doi: 10.1093/clinids/10.4.726. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Briese T., Chalkley L., Ellerbrok H., Kalliokoski R., Latorre C., Leinonen M., Martin C. Antigenic variation of penicillin-binding proteins from penicillin-resistant clinical strains of Streptococcus pneumoniae. J Infect Dis. 1991 Aug;164(2):313–319. doi: 10.1093/infdis/164.2.313. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Wachi M., Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990 Jan;141(1):89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Leyh-Bouille M., Ghuysen J. M. Isolation of the membrane-bound 26 000-Mr penicillin-binding protein of Streptomyces strain K15 in the form of a penicillin-sensitive D-alanyl-D-alanine-cleaving transpeptidase. Biochem J. 1982 Oct 1;207(1):109–115. doi: 10.1042/bj2070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras G., el Kharroubi A., van Beeumen J., Coeme E., Coyette J., Ghuysen J. M. Characterization of an Enterococcus hirae penicillin-binding protein 3 with low penicillin affinity. J Bacteriol. 1990 Dec;172(12):6856–6862. doi: 10.1128/jb.172.12.6856-6862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Van Landingham R. M., Walker L. L., Good R. C. Activity of selected beta-lactam antibiotics against Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1987 Jun;55(2):322–327. [PubMed] [Google Scholar]
- Spratt B. G., Zhang Q. Y., Jones D. M., Hutchison A., Brannigan J. A., Dowson C. G. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8988–8992. doi: 10.1073/pnas.86.22.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Cleavage of a COOH-terminal hydrophobic region from D-alanine carboxypeptidase, a penicillin-sensitive bacterial membrane enzyme. Characterization of active, water-soluble fragments. J Biol Chem. 1979 Jun 10;254(11):4863–4875. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Nanninga N. On the role of the high molecular weight penicillin-binding proteins in the cell cycle of Escherichia coli. Res Microbiol. 1991 Feb-Apr;142(2-3):333–344. doi: 10.1016/0923-2508(91)90049-g. [DOI] [PubMed] [Google Scholar]