Abstract
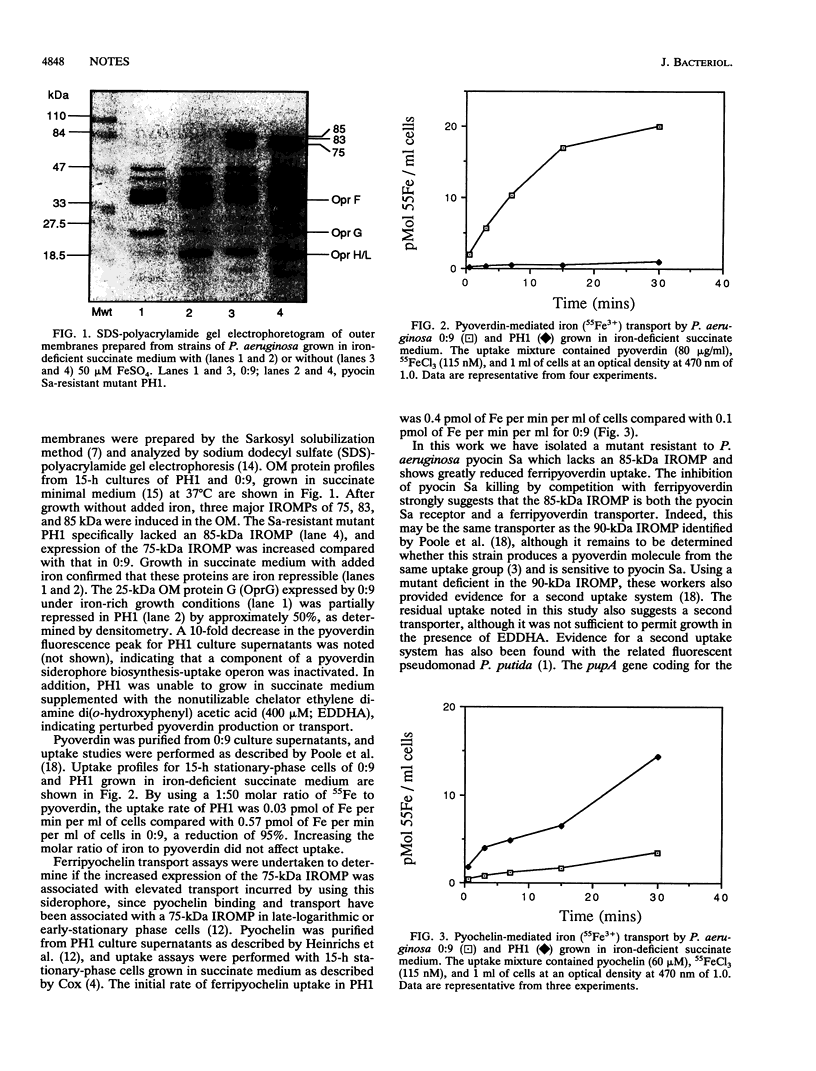
We have used Tn5 mutagenesis to obtain a mutant resistant to pyocin Sa. When grown in iron-deficient succinate medium this mutant lacked an 85-kDa iron-regulated outer membrane protein (IROMP), and expression of a 75-kDa IROMP was increased compared with that in the parent strain. The mutant was deficient in pyoverdin biosynthesis and showed a 95% decrease in transport of ferripyoverdin purified from the parent strain, suggesting that the 85-kDa IROMP is the specific receptor for ferripyoverdin and pyocin Sa. The mutant compensated for the deficiency in pyoverdin biosynthesis and transport by exhibiting a fourfold increase in ferripyochelin transport. The low-level transport of ferripyoverdin in the Sa-resistant mutant, which extended to heterologous pyoverdins from other strains, suggests that Pseudomonas aeruginosa has a second ferripyoverdin uptake system of lower affinity and broader specificity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Hohnadel D., Meyer J. M. Evidence for different pyoverdine-mediated iron uptake systems among Pseudomonas aeruginosa strains. Infect Immun. 1989 Nov;57(11):3491–3497. doi: 10.1128/iai.57.11.3491-3497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Harris G., Govan J. R. Revised pyocin typing method for Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jul;20(1):47–50. doi: 10.1128/jcm.20.1.47-50.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. Iron and bacterial virulence--a brief overview. Biol Met. 1991;4(1):7–13. doi: 10.1007/BF01135551. [DOI] [PubMed] [Google Scholar]
- Heinrichs D. E., Young L., Poole K. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect Immun. 1991 Oct;59(10):3680–3684. doi: 10.1128/iai.59.10.3680-3684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Kagiyama R. Biochemical relationship among three F-type pyocins, pyocin F1, F2, and F3, and phage KF1. J Biochem. 1983 Nov;94(5):1429–1441. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Hohnadel D., Khan A., Cornelis P. Pyoverdin-facilitated iron uptake in Pseudomonas aeruginosa: immunological characterization of the ferripyoverdin receptor. Mol Microbiol. 1990 Aug;4(8):1401–1405. doi: 10.1111/j.1365-2958.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Ohkawa I., Shiga S., Kageyama M. Effect of iron concentration in the growth medium on the sensitivity of Pseudomonas aeruginosa to pyocin S2. J Biochem. 1980 Jan;87(1):323–331. doi: 10.1093/oxfordjournals.jbchem.a132740. [DOI] [PubMed] [Google Scholar]
- Poole K., Neshat S., Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991 Feb;62(1):1–5. [PubMed] [Google Scholar]
- Sano Y., Kageyama M. Purification and properties of an S-type pyocin, pyocin AP41. J Bacteriol. 1981 May;146(2):733–739. doi: 10.1128/jb.146.2.733-739.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. W., Wilton J., Clark S. A., Alpar O., Melling J., Brown M. R. Production and characterization of monoclonal antibodies to outer membrane proteins of Pseudomonas aeruginosa grown in iron-depleted media. J Gen Microbiol. 1991 Feb;137(2):227–236. doi: 10.1099/00221287-137-2-227. [DOI] [PubMed] [Google Scholar]
- Sokol P. A. Tn5 insertion mutants of Pseudomonas aeruginosa deficient in surface expression of ferripyochelin-binding protein. J Bacteriol. 1987 Jul;169(7):3365–3368. doi: 10.1128/jb.169.7.3365-3368.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. M., Morris G., Brown M. R. Effect of iron concentration and growth rate on the expression of protein G in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1989 Apr;49(2-3):259–262. doi: 10.1016/0378-1097(89)90049-9. [DOI] [PubMed] [Google Scholar]